When we refer to crude oil as a raw material for the chemical industry, we are usually referring to crude oil, which a mixture of hydrocarbons. Strictly, we should be using the term petroleum, derived from Latin petra - rocks and oleum – oil. Petroleum describes not only the mixture of hydrocarbons in crude oil, including the gases and solids which are dissolved in the liquid but also any free gas, known as natural gas, associated with it.
- This unit describes how petroleum is formed and outlines the drilling techniques which are used to extract it.
- In another unit, the method of separating petroleum into separate fractions in a refinery by distillation is outlined.
- A third unit is devoted to the other processes used in in a refinery: cracking, isomerisation, reforming and alkylation. These processes produce gaseous and liquid fuels and the compounds needed in the chemical industry to make a vast number of products from plastics to medicines.
Petroleum that is worthwhile extracting is usually found trapped in layers of permeable rocks by other layers of impermeable rock, but more recently reserves of gas and oil are being extracted from shale which is an impermeable rock but is porous in the sense that there are spaces (pores) within its structure in which liquids and gases can be trapped.
Formation of natural gas and crude oil
Well over 200 different hydrocarbons can be identified in a sample of crude oil. They were formed in remote periods of geological time, anything from 50 to 500 million years ago, from the remains of living organisms. It is, therefore, a fossil fuel.
Weathered rock material, eroded from land masses and carried to the sea, accumulated in layers over millions of years in subsiding basins, and the remains of large quantities of marine plant and animal organisms became incorporated in the sediment (Figure 1).
Owing to the thickness of the sediments, high pressures built up which, probably in conjunction with biochemical activity, led to the formation of petroleum. The detailed mechanism is obscure, but it is probable that anaerobic microbes lowered the oxygen and nitrogen content of what had been living matter.
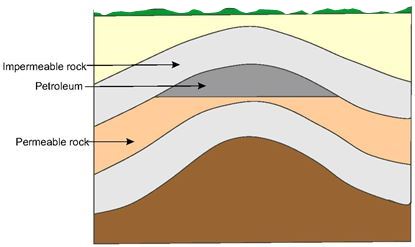
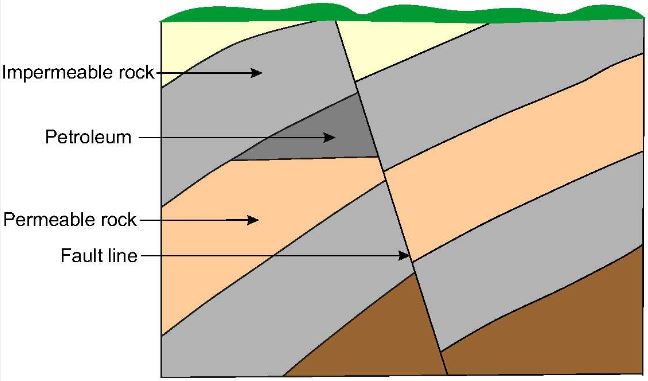
Subsequent earth movements which caused uplift of the sedimentary basins also caused migration of the petroleum through pores in the rocks, sometimes to areas far from where it was formed. In the course of migration, some of the petroleum accumulated in traps where the permeable rock was bounded by impermeable rock. The principal types of trap in oil fields found all over the world are the anticline (an upfold in the strata) as shown in Figure 1, the fault trap (Figure 2) and the salt dome (Figure 3).
 |
 |
|
| Figure 1 An anticline is where previously flat strata have been bent upwards by earth movements to form an arch. In this case the petroleum has migrated upwards in the permeable rock and become trapped by the overlying impermeable rock. | Figure 2 A fault line is the line along which the strata on one side has been displaced and is no longer aligned with the strata on the other side. In the example depicted here a layer of impermeable rock has trapped the petroleum by preventing it migrate further in the layer of permeable rock./span |
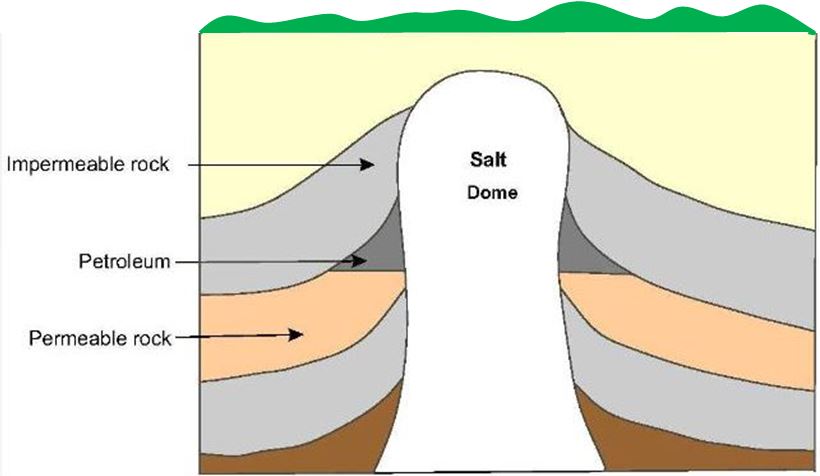 |
| Figure 3 Rock salt, when subjected to heat and pressure, can move very slowly upwards forcing its way through the overlying rock strata and so forming a salt dome. In the case shown, petroleum in the layer of permeable rock has become trapped by the overlying impermeable rock and the salt dome. |
Because the liquid oil and associated gas are trapped, in large amounts, into one area of permeable rock, it is possible to drill vertically into this rock and the oil and gas, under pressure rises up a pipe to the surface. The gas is separated from the oil and the crude oil is then said to be stabilized. The gas and oil are then transported by pipes either by land to a refinery or to a ship (tanker). If they are being transported by ship, the gas is liquefied before being pumped into the tanker. So that the tankers can readily offload the gas and oil, refineries all over the world are built near the coastline.
The liquid oil contains mainly alkanes (with 5 to about 125 carbon atoms in the molecules), cycloalkanes and aromatic hydrocarbons. The relative amounts of the three classes of compound vary with the oil-field, alkanes (15% - 60%), cycloalkanes (30% - 60%), aromatics (3% to 30%), with a residue of very high molecular mass hydrocarbons (e g bitumen) making up the remainder.
The average length of the carbon chains also varies from field to field. In some areas, there is preponderance of smaller hydrocarbon molecules (light crude oil) In heavy crude oil, there is a greater proportion of larger molecules.
Natural gas is principally methane, with smaller amounts of other alkanes, ethane, propane and the butanes. As with liquid oil, the composition of natural gas varies from field to field. In some fields, methane may make up 98% of the gas and it is known as dry natural gas. In wet natural gas, as much as 20% of the gas is made up of other alkanes, ethane, propane and the butanes. Some natural gas, as in Southern France, contains large amounts, up to 16%, of hydrogen sulphide, and others, as in the US, considerable amounts of helium. In some fields, the natural gas contains up to 7% of helium by volume.
Many of the oil fields are located off-shore which presents additional challenges.
Figure 4 The Mumbai High is an offshore oilfield 162 kilometres off the coast of Mumbai, India,
in about 75 m of water.
By kind permission of Nadu Chitnis (Wikimedia Commons).
| Figure 5 A pipeline being laid to connect to the Andrew Oil Field which is about 200 km north east of Aberdeen. By kind permission of BP. |
 |
|
Figure 8 The Lun-A (Lunskoye-A) drilling platform, located 15 km off the north eastern coast
of Sakhalin Island, on the east coast of Russia in a water depth of 48 m.
By kind permission of Dissident (Wikimedia Commons).
In the refineries, the gas and oil are separated by distillation into fractions with different boiling points which are then further processed (cracking, isomerisation, reforming and alkylation). Crude petroleum does not just consist of hydrocarbons. Also present are a variety of sulfur-containing compounds which must be removed during refining.
The organic sulfur compounds and hydrogen sulfide, both of which must be removed, for otherwise they will poison the catalyst needed in the manufacture of synthesis gas which leads to many of the most important industrial compounds. In the desulfurization unit, the organic sulfur compounds are often first converted into hydrogen sulfide, prior to reaction with zinc oxide. The feedstock is mixed with hydrogen and passed over a catalyst of mixed oxides of cobalt and molybdenum on an inert support (a specially treated alumina) at ca 700 K.
Then the gases are passed over zinc oxide at ca 700 K and hydrogen sulfide is removed:
Hydraulic fracturing (fracking)
Conventional natural gas and oil deposits are found in permeable rocks, trapped below impermeable rock. These deposits can be extracted by drilling down through the impermeable rock into the permeable rock.
But gas and oil are also trapped in the spaces within impermeable shale rock. Therefore, because shale is impermeable, simply drilling down to it is not enough to extract these deposits. Instead the process of hydraulic fracturing, known commonly as fracking, is used. The rock has to be fractured to get the gas or oil out.
Shale fields in the US were discovered in 1821 but the first use of fracking was 120 years later in the 1940s and it was not until this century that the development accelerated and there are now several hundred thousand shale wells in the US, with about 13,000 new wells being drilled every year.
While shale reserves are being explored world-wide, it is in the US where most fracking has occurred, and it is the only country to have such a large scale source of gas and oil which is commercially viable. One major example of a shale field is in North Texas (Dallas and Fort Worth) where the Barnett Shale stretches over 8,000 square miles and holds 86 trillion cubic feet of natural gas, enough to power all of homes in the US for almost 20 years. Other major fields in the southern states include Arkansas (Fayette shale), and Louisiana (Haynesville shale).
There are also very large shale areas in the Eastern states of the US. The largest is the Marcellus shale fields in Pennsylvania, Ohio and West Virginia. Others are in Illinois, Kentucky and Indiana (New Albany) and in Michigan (Antrim).
| Figure 9 There are very large shale areas across the US. This photograph was taken of a drill in the Marcellus shale field in Lycoming County in Pennsylvania. By kind permission of Rurhfisch (Wikimedia Commons). |
 |
|
 |
||
| Figure 10 And this photograph of drilling for shale gas and oil is on the other side of the US, near the Wind River Range in Wyoming. The Rocky Mountains can be seen behind the drill. By kind permission of the US Bureau of Land management (Wikimedia Commons). |
In conventional fields, the gas and oil is found free in large areas and so much can be obtained by drilling a bore vertically (Figure 1). Shale gas and oil is found in a large number of small pockets and a different technique is needed to get them to the surface, hydraulic fracturing.
This involves drilling down vertically 2 km or more below the surface before gradually turning horizontal and continuing drilling for as much as another 3 km. This allows a single surface site to accommodate the many small pockets of gas and oil.
Figure 11 Hydraulic fracturing (fracking) used to release oil and natural
gas from a stratum of shale.
The gap between the lining of the borehole that has been drilled and the surrounding rock is then sealed up with concrete to provide a secure route for the extraction of the gas and oil. There are small perforations in the horizontal portion of the well pipe, through which a mixture of water, sand and additives is pumped at high pressure (over 600 atmospheres) to create cracks (micro-fractures) in the shale for up to 50 metres. This fracking fluid is called slickwater. The sand (or other solid materials) are called proppants and are added to prop open the fractures that form under pressure. They are deposited in the fractures to keep them from closing up thereby ensuring that gas and oil can continue to flow freely out of rock fractures even after pumping pressure is released.
Up to 10 million litres of fracking fluid are pumped into the borehole under these extremely high pressures. When the pressure is released, the oil and gas can escape. A well head is then installed to capture the released oil and gas. The drilling and fracking equipment is then taken away.
A wide range of compounds, the additives, is also added to the water which serves a variety of purposes, from limiting the growth of bacteria to preventing corrosion of the well casing, friction‐reducing additives to allow fracturing fluids to be pumped along the pipe very rapidly, oxygen scavengers and other stabilizers to prevent corrosion of metal pipes (Table 1).
| Additive | Function | Examples of compounds |
|---|---|---|
| Biocide | Elimination of bacteria | quaternary ammonium salts |
| Acid | Dissolve some minerals and initiate fissure in the rock | hydrochloric acid |
| Friction reducer | Minimise friction between the pipe and the fluid | methanol, ethane-1,2-diol, polyacrylamide |
| Surfactant | lauryl sulfate salts | |
| Scale inhibiter | Prevent scale building up in the pipe | an inorganic phosphate |
| Buffer | Keeps the pH of the fluid constant | sodium carbonate, ethanoic acid |
| Corrosion inhibiter | Reduce corrosion of the pipes | methanol, propan-2-ol |
| Iron control | Prevents precipitation of iron oxides | citric acid, ethanoic acid |
| Cross linkers | Keeps the viscosity constant when the temperature of the fluid changes | boric acid, sodium borate |
| Gelling agents | Thickens the water to keep the sand in suspension | gums, methanol, ethane-1,2-diol |
Table 1 Additives: Example of compounds added to the water in hydraulic fracturing
From: ALL Consulting and is an updated version of the chart originally published in Modern
Shale Gas Development in the United States: A Primer, demonstrates the average volumetric
percentages of additives used for hydraulic fracturing treatment over multiple oil and gas plays.
The make‐up of a fracturing fluid varies to meet the specific needs of each area.
The flowback liquid contains water and contaminants, including the additives, but also radioactive material and heavy metals, hydrocarbons and other toxins. In the UNited States this wastewater is stored on the fracking site in pits, injected into deep underground wells or disposed of off-site at wastewater treatment facilities.
Figure 12 A fracking wastewater impoundment (pit) in the United Sates.
By kind permission of National Energy Technology Laboratory.)
The US government’s Environment Agency (EPA) has highlighted some concerns which include:
• Stress on surface water and ground water supplies from the withdrawal of large volumes of water used in drilling and hydraulic fracturing
• Contamination of underground sources of drinking water and surface waters resulting from spills and faulty well construction
• Adverse impacts from discharges into surface waters or from disposal into underground injection wells
• Air pollution from the release of volatile organic compounds, hazardous air pollutants and greenhouse gases.
From: www2.epa.gov/hydraulicfracturing
These concerns have been highlighted in recent years. Thus some US states (for example, New York) have not given permission for fracking while others are considering stronger regulations. There is also a study showing higher concentrations of hydrocarbons in the atmosphere near some fracking sites.
There are also concerns about harming the countryside particularly those areas deemed to be of particular natural beauty.
Fracking and the chemical industry
Throughout this website are examples of how the compounds separated from petroleum are used to manufacture the materials that we use every day. This section is devoted to how the gases liberated
by fracking are used in the chemical industry. The processes used to manufacture useful compounds from gas obtained by fracking are the same as those used to manufacture these compounds from petroleum obtained by conventional means. However, because the gases obtained by fracking are so much cheaper than those produced by other means, it is worth recalling the range of compounds that can be produced.
The composition of the gas varies between fields used for fracking (Table 2), just as it does in conventional fields, described above. Although this is a problem when a uniform composition is required, for example when the gas is used as a fuel, the presence of ethane, propane and butane is particularly welcomed by the chemical industry.
| Methane | Ethane | Propane | Carbon dioxide | Nitrogen | |
|---|---|---|---|---|---|
| Barnett Well 1 | 80.3 | 8.1 | 2.3 | 1.4 | 7.9 |
| Barnett Well 2 | 81.2 | 11.8 | 5.2 | 0.3 | 1.5 |
| Barnett Well 3 | 91.8 | 4.4 | 0.4 | 2.3 | 1.1 |
| Barnett Well 4 | 93.7 | 2.6 | 0.0 | 2.7 | 1.0 |
| Marcellus Well 1 | 79.4 | 16.1 | 4.0 | 0.1 | 0.4 |
| Marcellus Well 2 | 82.1 | 14.0 | 3.5 | 0.1 | 0.3 |
| Marcellus Well 3 | 83.8 | 12.0 | 3.0 | 0.9 | 0.3 |
| Marcellus Well 4 | 95.5 | 3.0 | 1.0 | 0.3 | 0.2 |
Table 2 Composition of natural gas (%) in the Barnett and Marcellus shale fields in the US.
From: K Bullin and P Krouskop Gas Producers Association Meeting Houston 2008.
Methane and ethane are separated from the other gases by fractionation. The mixture of propane and butane is known as liquefied petroleum gas (LPG) and much is used as a fuel. If needed as chemical feedstocks, propane and butane are separated by distillation.
Methane is the principal feedstock for synthesis gas and hence for chemicals such as methanol and ammonia.
Ethane is an important feedstock for ethene and hence a wide range of polymers, including poly(ethene), poly(chloroethene) and poly(phenylethene).
|
|
|
Figure 13 The first shipment from the US to Europe of ethane from shale gas was delivered to the petrochemical plant at Rafnes in Norway in March 2016 and the first to Grangemouth in Scotland in the following September. The ethane, which was stored at 283 K, was cracked to produce ethene (ethylene) and other alkenes. The Dragon, photographed here, is the largest ethane gas tanker in the world, holding 27,5000 m3 of gas. |
Propane is the main feedstock for propene, which in turn is used to produce polymers - poly(propene), acrylic polymers, poly(propenonitrile) – and cumene used to make phenol and propanone, epoxypropane, for the manufacture of polyurethanes.
This source of ethene, in particular, has given the US chemical industry a large advantage over other chemical industries around the globe. Its cost, over the recent past, is about a quarter that of ethene obtained from other sources.
Date last amended: 7th September 2018









