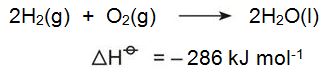![]() Hydrogen is one of the key starting materials used in the chemical industry. It is a fundamental building block for the manufacture of ammonia, and hence fertilizers, and of methanol, used in the manufacture of many polymers.
Hydrogen is one of the key starting materials used in the chemical industry. It is a fundamental building block for the manufacture of ammonia, and hence fertilizers, and of methanol, used in the manufacture of many polymers.
Uses of hydrogen
Hydrogen is used in the manufacture of two of the most important chemical compounds made industrially, ammonia and methanol. It is also used in the refining of oil, for example in reforming, one of the processes for obtaining high grade petrol and in removing sulfur compounds from petroleum which would otherwise poison the catalytic converters fitted to cars.
In years to come, hydrogen itself may become one of the most important fuels for cars as on burning it does not produce carbon dioxide, but there are major problems to be overcome before it can be used in this way. These include its manufacture, storage, distribution and how it can be used efficiently in cars.
.jpg)
Figure 1 Uses of hydrogen.
Annual production of hydrogen
| World | 50 million tonnes |
Manufacture of hydrogen
By far the most important process for making hydrogen is by steam reforming.
The key parts of the process are the conversion of a carbon-containing material to a mixture of carbon monoxide and hydrogen followed by the conversion of carbon monoxide to carbon dioxide and the production of more hydrogen.
At present, the hydrocarbon used is generally methane or other light hydrocarbons obtained from natural gas or oil and coal. However, there is an increasing interest in using biomass as outlined in the unit on biorefineries.
If hydrocarbons are used, the gas or vapour is mixed with a large excess of steam and passed through pipes containing nickel oxide (which is reduced to nickel during the reaction), supported on alumina, in a furnace which operates at high temperatures:
.jpg)
The reaction is endothermic and accompanied by an increase in volume. It is thus favoured by high temperatures and by low partial pressures. The reaction is also favoured by a high steam:hydrocarbon ratio. This increases the yield but increases operating (energy) costs. The high ratio also helps to reduce the amount of carbon deposited which reduces the efficiency of the catalyst. The most effective way to reduce carbon deposition has been found to be impregnation of the catalyst with potassium carbonate.
In the second part of the process, the shift reaction, carbon monoxide is converted to carbon dioxide by reacting it with steam and so producing more hydrogen:
.jpg)
This reaction is significantly exothermic, and so high conversions to carbon dioxide and hydrogen are favoured by low temperatures. This is difficult to control due to the heat evolved, and it has been common practice to separate the shift reaction into two stages, the bulk of the reaction being carried out at around 650 K over an iron catalyst, and the 'polishing' reaction carried out around 450 K over a copper/zinc/alumina catalyst.
The carbon dioxide and any remaining carbon monoxide are then removed by passing the gases through a zeolite sieve. From time to time, the vessel containing the sieve is taken out of the gas stream and flushed with hydrogen to displace carbon dioxide and regenerate the sieve.
To obtain a sustainable (i.e. zero emission of greenhouse gases) production of hydrogen, the carbon dioxide is captured and stored or used.
Thus, overall, one mole of methane and two moles of steam are theoretically converted into four moles of hydrogen, although this theoretical yield is not achieved as the reactions do not go to completion:
.jpg)
In some countries (notably China) methane and other gases based on oil are in short supply and need to be imported. This has led to a significant change in the choice of fuel used in reformers in these countries. Instead of a hydrocarbon gas, coal is used as it it is more available.
Research is being undertaken to see whether biomass instead of coal or oil can be used effectively to manufacture hydrogen. One of the key issues is to minimise the energy used in collecting the biomass and transporting it to the place of use, which may involve the use of non-renewable energy sources. Financial and environmental costs can be high, relative to the savings in switching from non-renewable resources to biomass.
The future: 'The hydrogen car'
Hydrogen is potentially an environmentally attractive fuel for the future. When it burns to produce energy, the only product is water:
There are two key points to consider:
(a) How to manufacture hydrogen in as ‘green’ a way as possible.
(b) How to use the gas efficiently to produce energy.
(a) Manufacture of hydrogen
Hydrogen, as discussed above, is produced on a large scale from fossil fuels (natural gas, coal), thus also producing the environmentally damaging greenhouse gas, carbon dioxide.
The obvious route to produce hydrogen is by the reversing the process of burning hydrogen to produce water, that is the electrolysis of water. The overall equation is:
.jpg)
However, this needs electricity from power stations. If the power station uses fossil fuels, it defeats the purpose, namely to produce a fuel without the production of carbon dioxide. Other forms of generating power, such as nuclear, wind and geothermal, do not have this disadvantage but these routes are not readily available in many countries.
However the hydrogen is produced, there are problems in distributing the gas in an economic but environmentally-friendly way.
In the existing supply network, hydrogen is delivered under pressure to hydrogen refueling stations (HRS) by specially constructed tankers either as liquid hydrogen or as compressed gaseous hydrogen and then transferred into storage vessels. An alternative and cleaner method is via a pipeline and there are small existing pipeline networks, for example, in Germany, the Netherlands and California which are used to deliver hydrogen straight from the point of manufacture.
Hydrogen refuelling units are being developed which produce hydrogen in situ in the garage. By the end of 2016, it is expected that there will be several hundred such refuelling sites in the US, Germany, Japan and the UK which will be able to deliver hydrogen to a car at pressures between 350 and 700 atmospheres.
|
Figure 2 This shows an electrolyser constructed on two levels in a special 4 m compartment within a garage. It can produce 100 kWh worth of hydrogen fuel per day. The post on the upper level is the ionic compressor which enables the hydrogen to be delivered to a car at an appropriate pressure. |
 |
As noted above, there are practical difficulties in the distribution and storage of hydrogen. One approach would be to convert a liquid fuel into hydrogen, in situ in the car. For example, methanol has been used in experimental cars. The fuel was converted into hydrogen and carbon dioxide by a reforming reaction, similar to the process described above for the large scale manufacture of hydrogen. This demands a very high level of engineering skills to produce conversion units which are light enough for a car but strong enough to withstand all the problems caused by continuous vibrations.
An enormous amount of research is also being undertaken on using sunlight as the energy source, one being via biophotolysis.
This involves the production of algae in water through photosynthesis, followed by bacterial decomposition of the algae to produce hydrogen. An important discovery was that by depriving the algae of sulfur, normal photosynthesis is inhibited and instead an enzyme is activated and hydrogen, not oxygen is produced in light. Present research is concerned with making these processes more efficient.
(b) How to use hydrogen efficiently in engines to produce energy.
At a hydrogen fuel station the hydrogen fuel is safely transferred to the hydrogen fuelled car by means of a specially designed nozzle.

Figure 3 A hydrogen fuelling nozzle.
By kind permission of The US Office of Energy Efficiency
and Renewable Energy (EERE)
Hydrogen could be burnt in an engine in a similar way to petrol, the gas burning in air to release energy. Although there is an advantage over using a hydrocarbon as there is no carbon dioxide produced, harmful nitrogen oxides would still be formed, by the reaction of nitrogen and oxygen in the hot engine.
However in a fuel cell, hydrogen reacts with oxygen without burning. The energy released is used to generate electricity, which is used to drive an electric motor. This is a more environmentally friendly system and over the last decades much research has been undertaken by chemists and engineers to produce very efficient fuel cells where about half of the energy from the reaction between hydrogen and oxygen to produce water is released as an electrical potential.
| Figure 4 A Mercedes-Benz B-Class F-CELL car which uses hydrogen as the fuel. By kind permission of Daimler AG |
 |
One such fuel cell is the PEM cell, where PEM stands for Proton Exchange Membrane or Polymer Electrolyte Membrane.
All Fuel Cell Electric Vehicles (FCEVs) use pure hydrogen as a fuel which is stored in pressure tanks mainly at 700 atmospheres and a stream of pure hydrogen is delivered to the anode on one side of a membrane, which allows movement of cations but does not conduct either anions or electrons. One such material is a co-polymer of a sulfonated tetrafluoroethene (tetrafluoroethylene), incorporating perfluoroethenyl (perfluorovinyl) ether groups terminated with sulfonate groups onto a tetrafluoroethene backbone. The H+ ions (protons) are able to pass through the membrane as the proton on the -SO3H (sulfonic acid) groups "hop" from one acid site to another.
.jpg)
Figure 5 Structure of a membrane cell.
Thus, protons are formed:
.jpg)
The electrons pass through the external circuit from the anode to the cathode and so create an electrical potential. The protons permeate the membrane and react with oxygen at the cathode:
.jpg)
Both reactions are catalysed by platinum. As platinum, is extremely expensive, it is deposited as nanosized platinum particles onto carbon powder (Pt/C) which provides a large platinum surface area while the carbon allows for electrical connection between the catalyst and the rest of the cell. Platinum is so effective because it has high catalytic activity and bonds to the hydrogen just strongly enough to facilitate electron transfer but not inhibit the hydrogen from continuing to move around the cell.
The most effective ways of achieving the nanoscale platinum on carbon powder use chemical solution deposition. The platinum particles are deposited onto carbon paper that is permeated with PTFE. A second method of increasing the catalytic activity of platinum is to produce an alloy with other metals, such as nickel. It is thought that this reduces its tendency to bond to oxygen-containing ionic species thereby increasing the number of available sites for oxygen adsorption and reduction.
The reactions take place at ca 350 K and the process is therefore referred to as ‘cold combustion’ in a low-temperature fuel cell.

low potential of less than 1 volt, several hundred cells are connected in series to form a
so-called stack. The system potential thereby attained, amounting to 200 volts, is
sufficient to power a vehicle.
By kind permission of Daimler AG
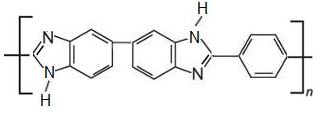
Other, more recent membranes include polymers based on polybenzimidazole (PBI), which are stable at temperatures at about 500 K. At these higher temperatures, the membranes are more efficient and stronger although there can be problems with their manufacture.
polybenzimidazole
Polybenzimidazole itself is produced as a fibre which has a very high melting point. It is both highly resistant to heat and to chemicals and has been used successfully to make a material used by firemen, astronauts and others exposed to danger from flames and other sources of high temperatures.
There is underway much research and development into how to use hydrogen as a fuel. In particular, progress has been made in the development of hydrogen based fuel cells but it is clear that the use of these cells to power cars that are to be used in conventional ways does present significant challenges associated with the creation of the hydrogen and its transport, storage and distribution. Some of these difficulties are by-passed where cells are used to power transport vehicles within a business site that is large enough to produce, store and distribute its own hydrogen to its own vehicles that work on the site and emit only water.
Anoher approach would be to convert a liquid fuel into hydrogen, in situ in the car. For example, methanol can be converted into hydrogen and carbon dioxide by a reforming reaction, similar to the process described above and used in a fuel cell but today no car manufacturer is working on such reformer systems for fuel cell electric vehicles (FCEV). However, for stationary applications of fuel cells, reforming of hydrocarbons is reasonable and projects are underway around the world to convert natural gas to hydrogen in mini-reformers.
Date last amended: 27th July 2016