The products of the chemical industry are used to produce objects that vary enormously in their size from say the iron girders for bridge building to silicon chips in microprocessors. However, techniques are now available which make it possible to manipulate materials on the atomic or molecular scale to produce objects which are no more than a few nanometres in diameter. A nanometre is 1 x 10-9 metres (a billionth of a metre). This is more than a 1000 times smaller than a silicon chip.
The processes used to make and manipulate such materials are known as nanotechnology and the materials or objects themselves are called nanomaterials.
|
| Nanotechnology is now used in chemistry, physics, biology and engineering. The smallness of the particles confers on them very useful properties. Some of these properties arise from the enormous increase in the surface area when, for example, a powdered material is converted into particles which are a few nanometres in diameter. This increase in surface area will lead to an increase in the rate of any reactions which occur on the surface of the material. The small dimensions of nanomaterials also lead to the possibility of them forming intimate mixtures with other materials with a view to enhancing the properties of the material. In medical treatments they can be tailored to provide opportunities to target some medications more precisely. |  |
| Figure 2 Even the finest hair on this fly has a diameter of about 5000 nanometres. By kind permission of Bruce G Marcot. |
As can be seen in Figure 3, materials or objects that need to be measured in nanometres have always existed but the techniques for manipulating materials on this scale have only been developed during the last twenty years or so.
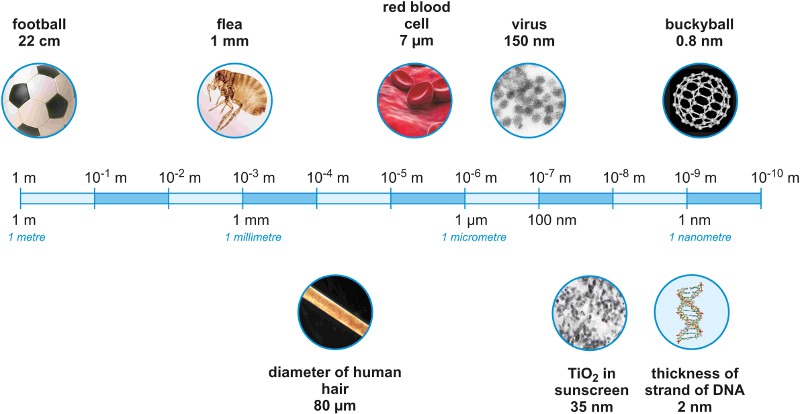
Figure 3 A scale to show the relative dimensions of various objects.
By kind permission of Dr Alan Smith.
The invention and development of the scanning tunnelling microscope (STM) in 1981 at the IBM Laboratories in Switzerland has essentially provided the basic technology for work on the nanoscale. By scanning the surface of materials, it has not only become possible to visualise individual atoms and molecules, but we can even pick them up and move them around. One of the defining moments was in 1989, when IBM scientists used STM to spell out the letters I-B-M in individual xenon atoms on a nickel crystal surface.
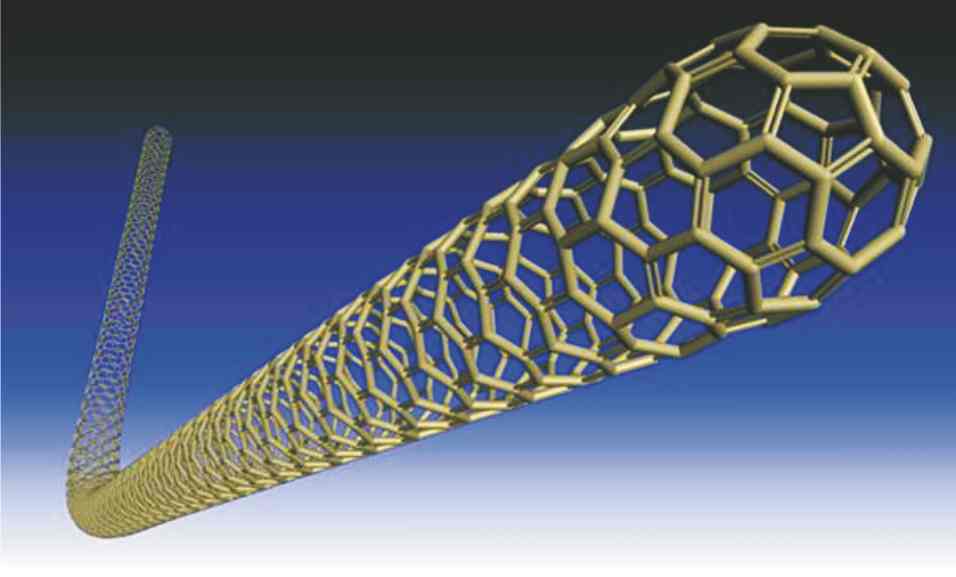
A variety of materials have led to the burgeoning use of nanoparticles to change and improve the properties of products. Clay structures are made up of nanoparticulate platelets and are being used in composites to reduce weight and improve strength, but the most exciting developments have come from allotropes of carbon. This was initiated by the discovery of buckminsterfullerene, C60, (affectionately known as buckyballs) in 1985 (Figure 4), and the subsequent production of carbon nanotubes (Figure 5). However, graphene (Figure 6) has been heralded as a ‘wonder’ material. It was first produced by removing each layer of graphite with sellotape. Graphene is about 200 times stronger than steel, yet incredibly lightweight and flexible, but in addition, it is transparent, thermally conductive, and is even more electrically conductive than copper.
|
|
|
|
Figure 4 A computer-generated model of a buckyball (buckminsterfullerene, C60) molecule. |
Figure 5 A computer-generated model of a carbon nanotube. |
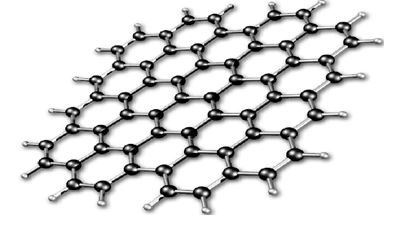 |
| Figure 6 A single sheet of graphene. By kind permission of Google |
Manufacture
Nanomaterials can either be created by cutting down macro structures to the nanoscale (top-down approach) or by assembling structures from atoms and molecules (bottom-up approach).An example of the top-down approach is seen with the manufacture of microprocessors, in which shortwave ultra-violet and electron beams are used to cut silicon wafers that are used to produce 'circuits' with nanoscale structures (less than 50 nm). This approach, however, tends to be very wasteful of expensive materials that are etched away during the process.
Building up materials atom by atom, or molecule by molecule, creates less waste. The nanostructures are formed because each atom or molecule recognises its natural position, similar to ions building crystalline structures from solution. Continuous production of nanomaterials in bulk is already established up to tonnage quantities. Among the methods used to produce them are physical and chemical vapour deposition.
In physical vapour deposition (PVD), the material is vaporised by heat in a furnace or by pulsed lasers. The vapour is then condensed on a cool surface. For example, single-wall carbon nanotubes can be prepared by vaporising a carbon target in a furnace at about 1500 K using a laser and allowing the vapour to condense on a cool surface. An inert gas is bled into the reactor during the process to prevent oxidation of the carbon vapour.
In chemical vapour deposition (CVD), a reaction occurs in the vapour phase between two or more materials and/or the vapour reacts with the target material.
This bulk production technology has been used to manufacture a wide range of materials in 20-100 nm particle size ranges (nanopowders). For example, compounds used in the electronics industry, such as silicon dioxide (from silicon hydride and oxygen) and silicon nitride (from silicon hydride and ammonia), are made in this way. Some metals (for example, nickel and tungsten) of nano size are also prepared by CVD, by reducing their chlorides with hydrogen at high temperature.CVD is thought to show the most promise for the production of carbon nanotubes (Figure 7), and the same method can be used to produce graphene, but other methods are also being developed.
.jpg) Figure 7 A line diagram illustrating chemical vapour deposition.
Figure 7 A line diagram illustrating chemical vapour deposition.
By kind permission of Dr Marcello Motta, Thomas Swan and Co Ltd
Uses
In this section, a wide range of uses of nanomaterials is discussed, some of which are currently in production and others are in an advanced state of development.
| Figure 8 The glass in the Lycurgus Cup (probably made in Rome in the 4th Century AD) and now in the British Museum contains nanoparticles of silver and gold. It is coloured green but when held up to the light, the colour changes to glowing red. By kind permission of the Trustees of the British Museum |
 |
Sports and leisure
Sports and leisure applications always seem to be the first to accept new technologies and nanotechnology has not been an exception
Carbon nanotubes produce composites with epoxy resins with a tensile strength 5 -10 times higher than comparable carbon fibre reinforced materials. Yacht masts have been produced commercially, which are up to 30 times stiffer without adding any extra weight.
| Figure 9 Nano-dispersed clays and butyl rubber (poly(2-methylpropene)) provide an excellent seal for air. The inside of tennis balls can be sprayed with a solution containing them and this makes the tennis balls much more air tight than the usual tennis ball and will maintain the air pressure, and hence their bounce, for longer. By kind permission of Dr Alan Smith. |
 |
Other applications of carbon nanotube reinforced composites include tennis rackets, fishing rods, and car body panels used in racing cars for strength and lightness, thereby enhancing performance. The cushioning properties of trainer shoe soles have been improved by incorporating nanoparticles thereby changing the structure of the polymeric soles, whilst at the same time extending durability because of the hardness enhanced by the presence of the nanoparticles.
Sports company, HEAD, uses graphene in their new tennis rackets, which provides a 30% increase in strength, and they are up to 20% lighter compared to a conventional racquet with the equivalent swing weight. The same company has incorporated graphene into snowboards, skis, and squash rackets to take advantage of the improved properties

Figure 10 HEAD snowboard incorporating graphene
By kind permission of Google
Graphene has also been incorporated into golf balls to improve the golfer's game by delivering faster ball speed, high launch, and low spin, to give longer distance.

Figure 11 Callaway’s new graphene golf ball.
By kind permission of Dr Alan Smith.
Graphene-enhanced rubber has been developed for the outsoles for running and fitness shoes that in testing have outlasted 1,000 miles and are scientifically proven to be 50% harder wearing.

Figure 12 Innov 8 Running shoes
By kind permisson of XXXX
Transport
The Airbus A380 uses about 20% composite materials containing nanomaterials, saving fuel through weight reduction. Increasing the miles per gallon for cars, aircraft, ships, and any form of transport is not only energy saving, but also reduces costs dramatically. Nanotechnology has been very active in improving the environmental impact of many forms of transport. For example, the cost of carrying 1kg extra in weight can cost up to $300 in fuel over 5000 flying hours. This is the reason why some airlines even use lightweight paper for their in-flight magazine to reduce weight as much as possible. By using tablet computers instead of heavy log books, one airline has reported savings of over $300,000 per year.

Figure 13 Virgin Atlantic estimates that shaving a single pound off all the planes in their fleet would
save them 14,000 gallons of fuel per year. The airline has redesigned its meal trays fitting more smaller,
lighter trays on each meal cart, which means fewer meal carts per plane. The net result is close to a 300-pound weight loss.
By kind permission of Google.
The ability of nanoparticulates to reduce weight and increase strength, whether by using nano-fibres or more recently graphene, is making a significant contribution to energy savings.
Utilities
Nanotechnology is actively being developed in several energy generation applications. Solar panels use nanoparticles to help improve their efficiency. For wind turbines, the larger the blade the more electricity can be generated, so nanocomposite materials which are stronger and weigh less are being increasingly used to avoid breakages of the blades.
In the water sector, new research at Manchester University has shown that graphene can filter common salts from water to make it safe to drink. These findings could lead to affordable desalination of sea water.
Health and personal care
Nanotechnology is having an enormous impact in the healthcare and personal care industries, because of the extremely small dimensions of nanoparticles and their mobility. The chemical reactivity rate, the location of effect, and the timing of a treatment are all affected by particle size. Efficient drug delivery is being tested already. Biological microelectromechanical devices (bioMEMS) implanted into the body to deliver doses of drugs or carry new cells to damaged tissues bring the concept of nanosurgery into being.
In the area of biomedical imaging, the use of nanoparticles as image enhancers is being developed. Imaging probes and implant coatings can be inserted into the human body with particle sizes from 2-10 nm. The enhanced magnetic properties of iron(III) oxide nanoparticles make them suitable for use as contrast agents in magnetic resonance imaging (MRI).
In the study and medical treatment of cancers, nanocarriers can be used for delivering imaging agents to cancer cells thus making it easier to locate the cancer cells precisely and making treatment much more effective. One technique being tried is to inject the patient with certain nanoparticles, often gold because of its resistance to corrosion. The gold nanoparticles that are located at a site of cancer cells can be irradiated with infrared to heat them up and destroy the nearby cancer cells.
Cosmetics are already available that contain nanoparticles, again exploiting properties arising from their incredibly high surface areas. These include sun lotions and anti-aging creams.
|
Figure 14 Silver nanoparticles provide powerful antiseptic properties and are used, for example, in baby food cartons to prevent cross-contamination and in fabric dressings. |
 |
Nanoscale titanium dioxide, containing very small quantities of manganese(II) oxide, is being used in sun lotions as the mixture absorbs harmful UV radiation. The nanoparticles are so small that they are invisible to the naked eye and the lotion appears clear. The nanoparticles are stable to light and work by reducing the harmful materials (free radicals), formed in the skin from exposure to the sun, that lead to premature skin aging and skin cancers. Claims are being made for the effectiveness of nanoparticles in anti-aging preparations to give protection from harmful UV radiation and they can also be used to deliver vitamins that plump and soften the skin, thereby reducing wrinkles. Zinc oxide, zinc and silver nanoparticles are being used for their anti-microbial and anti-bacterial properties in some skin preparations.
Nanoparticles of copper(I) oxide, displaying anti-fungal properties, are being incorporated into coatings, fibres, polymers, bandages, plastics and soaps.
A gecko’s ability to scurry around upside down on ceilings is all down to nanotechnology. The ‘nano-hairs’ on each toe are attracted to the surface by van der Waals forces, and when there are millions of them the creature can hold on quite easily. This adhesion is being used for plasters and wound dressings which stick well but do not leave sticky marks when removed.
A company called nanoGriptech is exploiting this technology for a wide variety of ‘dry’ adhesives, including goalkeepers gloves so the goalie can grab hold of the ball more easily.
Some of the most exciting nanotechnology work being carried out in the medical sector is on sensors, which have the ability to detect diseases before they have taken a hold on your body. Such detection is not dissimilar to a dog’s sense of smell, where for example in drug detection they are only sniffing out a few molecules. Many nano-sensors use small electronic detectors to identify changes, and some companies are actually detecting specific diseases on people’s breath.

Figure 15 Based on the gecko’s grip, it is possible to purchase coat hooks that
stick to most surfaces based on the nano-structure of the gecko’s nano-hairs.
By kind permission of Dr Alan Smith.
A company called nanoGriptech is exploiting this technology for a wide variety of ‘dry’ adhesives, including goalkeepers gloves so the goalie can grab hold of the ball more easily.
Health and safety and the environment
There has been much debate over the safety and environmental impact of nanotechnology. Concerns have been expressed regarding nanoparticles as to whether their small sizes and novel properties may pose significant health or environmental risks.
As many of the applications described above involve nanoparticles being locked into polymeric composites, film and fibres, it is not expected that such nanomaterials will pose a threat to the health and safety of users. As with most chemical compounds, the exposure of plant operators handling 'raw' nanoparticles must take precautions to avoid skin contact and inhalation.
On the other hand, some global environmental issues might well be resolved using nanomaterials. One of the greatest potential impacts of nanotechnology on the lives of the majority of people on Earth could be desalination and purification of water, providing fresh water from the oceans and from brackish wells. Research is progressing on membranes into which graphene is embedded to add to the strength of the membrane and make it more efficient. Again because of the high surface areas, nanomaterials, such as some silicates, make excellent filters for trapping heavy metals and other pollutants from industrial wastewater. As already described, energy saving with nanotechnology-based applications is receiving a lot of attention.
The future
Nanotechnology is already having an impact in many spheres of chemical and materials science. It would seem that only our imagination will limit the widespread application of nanotechnology. The global market for nanocomposites totalled $2.0 billion in 2017 and is estimated to reach $7.3 billion by 2022.
Date last amended: 14th March 2019