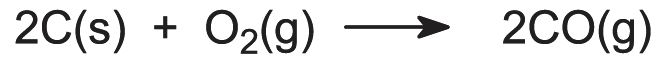Uses of iron
A very small proportion of iron is used as cast iron. It is about 92% pure and contains some carbon (2-5%) which makes it brittle, together with smaller quantities of silicon (1-3%), manganese, phosphorus and sulfur, as impurities. It was traditionally used for products like heating radiators, fireplaces, gutters, bollards and lamp posts. However, due to its brittleness and tendency to rust, it has been replaced by other materials for most of these uses, although cast iron objects are still made for their aesthetic qualities.
Wrought iron contains less than 0.15% carbon and was made by reducing iron ore in the solid state, and then hammering to remove the slag. Because it could be bent and hammered into shape, it was used for 'traditional' gates, garden furniture and other decorative products. These are now made mostly from steel, and genuine wrought iron is no longer produced in any quantity.
Annual production of iron
Rapid economic growth in China has been accompanied by an expansion in the iron and steel industries. Almost 50% of all the iron produced in the world is made in China, with about 1% produced in the UK.
| World | 1180 million tonnes |
| China | 710 million tonnes |
| Japan | 84 million tonnes |
| India | 54 million tonnes |
| Russia | 51 million tonnes |
| Korea | 47 million tonnes |
| Brazil | 30 million tonnes |
| Germany | 28 million tonnes |
| US | 26 million tonnes |
Data from:
U.S. Geological Survey, Mineral Commodity Summaries, 2016.
Manufacture of iron
Iron is produced by reduction of iron ore, which is often a mixture of oxides, using carbon, carbon monoxide, and hydrogen. While the blast furnace is the dominant reduction process other technologies are emerging which operate on a smaller scale. These are linked to locations where there is a plentiful supply of natural gas or low-grade coal.
The manufacture of iron has two stages, the preparation of raw materials, and the reduction of iron oxide to iron.
(a) Preparation of raw materials
Iron is one of the most abundant elements on Earth and its ores commonly contain oxygen, silicon, manganese, phosphorus and sulfur. The major minerals present in ores include haematite (Fe2O3) and magnetite (Fe3O4). Much of the ore is mined in Australia, Brazil, China, India, Russia and the US.
Most ores contain more than 60% iron and are used in their mineral state in a blast furnace. Ore containing less than this is first crushed and ground into a powder and concentrated by flotation. It is then rolled into balls and heated in a furnace to produce pellets the size of marbles. This process takes place near the mine, reducing the long-distance transport of waste material (clays and other silicates).
Coke, a porous solid, provides carbon for the reduction reactions and is also the main fuel used in the furnace. It is made on site by heating coal to ca 1200 K in the absence of air for up to 20 hours in a battery of coke-ovens. The residue is coke, and a range of volatile compounds is driven off. A gas, coal gas (mainly carbon monoxide and hydrogen), and a black tar from which useful compounds such as benzene can be obtained, are also produced. Coal gas is used as a fuel on site.
Figure 1 Iron ore and coal waiting to be used in a blast furnace in Ijmuiden near Amsterdam in the Netherlands.
By kind permission of World Steel Association.
Oxygen is required for the burning of fuel (to create a high temperature) and also takes part in the furnace reactions. In order to help maintain the furnace temperature, the air is passed through a pipe which circles the furnace (a bustle pipe) and then through nozzles (tuyeres) into the furnace (Figure 3) at about 1500 K. Many furnaces now use oxygen-enriched air, which reduces the amount of gases passing through the furnace and also ensures the reactions are complete.
In some furnaces oil or natural gas is injected with the pre-heated air, replacing up to 40% of the coke. This reduces both dependence on coal and the need to process by-products from the coking ovens, which may be difficult to sell. Alternatively, finely powdered coal may be injected directly as a fluid into the furnace, eliminating the need for coking ovens. Some manufacturers are experimenting with using waste wood or plastics as a fuel.
(b) The reduction of iron oxide to iron (The Blast Furnace)
Blast furnaces are major engineering constructions, part of which is a steel cylinder up to 30 m high, lined with special bricks capable of withstanding very high temperatures. These are also water-cooled. The widest point of the furnace, the hearth at the bottom, is typically 9 m in diameter, although it can be larger. Furnaces operate more or less continually for up to 15 years at pressures up to 5 atmospheres, and internal temperatures in excess of 2000 K. They can produce as much as 10 000 tonnes of molten iron per day, up to 50 million tonnes over the lifetime of the furnace.
|
The blast furnace uses either high-grade iron ore or the iron ore pellets together with coke and limestone. In a modern blast furnace, the mass of each component and the timing of its addition to the furnace are computer-controlled, responding automatically to the conditions prevailing at that time in the furnace. The components are added in small amounts every 10-15 minutes at the top of the furnace.
A pressure of ca 1.7 atm is allowed to build up in the furnace, giving better combustion of the coke and other fuels and a higher output of iron.
Hot oxygen-enriched air is blown in near the bottom, the pipes being known as tuyeres (Figure 3). Many reactions take place as the gases make their way upwards.
Coke reacts with oxygen in the blast to form carbon monoxide, a reducing agent:
If oil or natural gas is used, the hydrocarbons produce a second reducing agent, hydrogen:
Figure 3 A blast furnace for reducing iron ore to iron.
Temperatures vary within the furnace, the highest temperatures being at the bottom and the lowest at the top, and so the various reactions take place at different levels in the furnace.
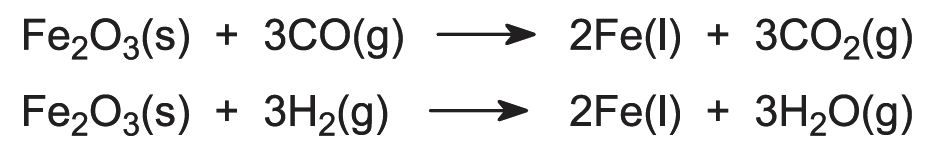
Near the top of the furnace, at about 750 K (below the melting point of iron), iron(III) compounds are reduced to iron(II) (for example Fe2O3 to FeO) by carbon monoxide and hydrogen. Lower in the furnace, where it is hotter, the reduction to iron is completed.
The overall equations for the reduction can be expressed as:
The molten iron runs down and collects in the bottom of the furnace. As it descends it absorbs carbon, phosphorus, sulfur and small amounts of other elements such as manganese and silicon from the ores, coke and limestone.
In regions of the furnace where the temperature is higher than 1150 K limestone dissociates, producing calcium oxide:
Calcium oxide, which is a base, reacts with acidic impurities in the ore, forming an aluminosilicate slag. This also absorbs much of the sulfur present in the various raw materials. The liquid slag runs to the bottom of the furnace, forming a layer on top of the molten iron.
The molten iron (of 90-95% purity, the main impurity being ca 4% carbon) and liquid slag are removed from the hearth through tapholes at the base of the furnace.
Usually the molten iron is sent directly to the steelworks as 300 tonne loads in refractory-lined, torpedo-shaped transfer ladles.
|
The slag is run off, every few hours, cooled and then sent for treatment to make by-products such as cement and insulation products or to be used for road building.
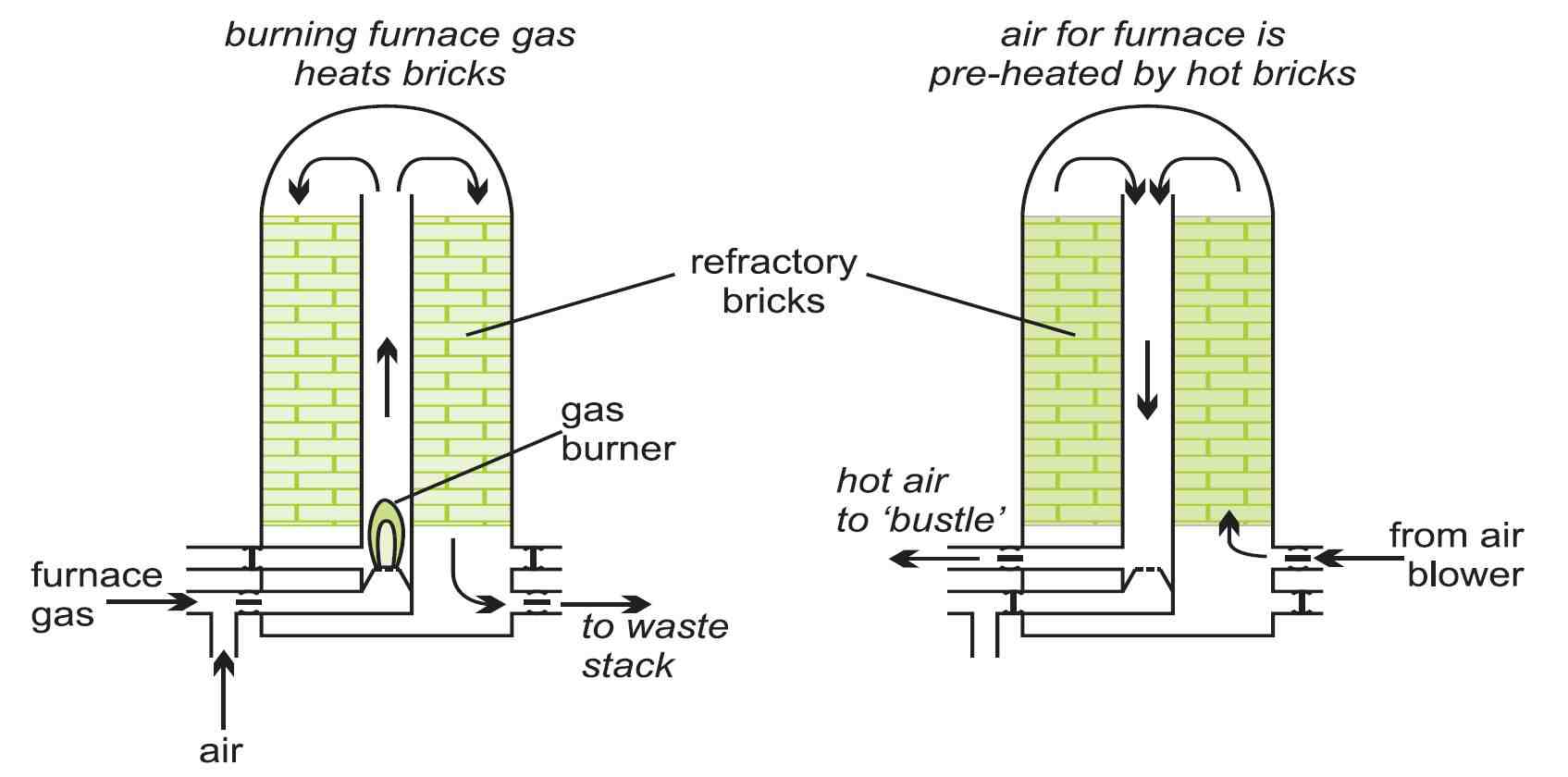
The gas emerging from the top of the furnace contains nitrogen, carbon monoxide, carbon dioxide, hydrogen, water vapour and dust. After removing the dust, the gas, mixed with natural gas, is used as a fuel to heat bricks packed into 'stoves'.
Figure 5 Brick stoves use the heat from the waste furnace gases to preheat the
incoming air prior to it passing through the bustle pipe
and the tuyeres into the blast furnace.
The heat from these stoves is used to pre-heat the air blast. These energy-saving measures have an important impact on the overall economics of blast furnace operation. Other measures include using oxygen-enriched air in the blast, the use of hydrocarbons as auxiliary fuels, operating the furnace at a higher pressure, minimising the use of limestone, and preparation of the raw materials so that the chemical processes within the furnace take place more rapidly and use the minimum amount of fuel.
Removal of sulfur
|
Some steels need a very low concentration of sulfur, which can make it brittle and can lead to structural failure. Unlike other impurities which are removed from the hot metal by oxidation in the oxygen converter, the most economic method of removing sulfur from the hot iron is prior to the steel-making process. It is done by adding a reagent. Often lime is used but magnesium is many times more effective. The reagents, with nitrogen as a carrier gas, are injected well below the surface of the molten iron, a process known as deep injection. For example: Sulfur-rich slag generated during the process is removed by rapidly skimming off the scum as it forms. Figure 6 The One World Trade Centre in Lower Manhattan, New York City, uses over 40 000 tonnes of high structural steel The tallest building in the Western hemisphere is seen here at sunset. By kind permission of Marco Vetch (Wikimedia Commons) |
 |
Date last amended: 3rd October 2016