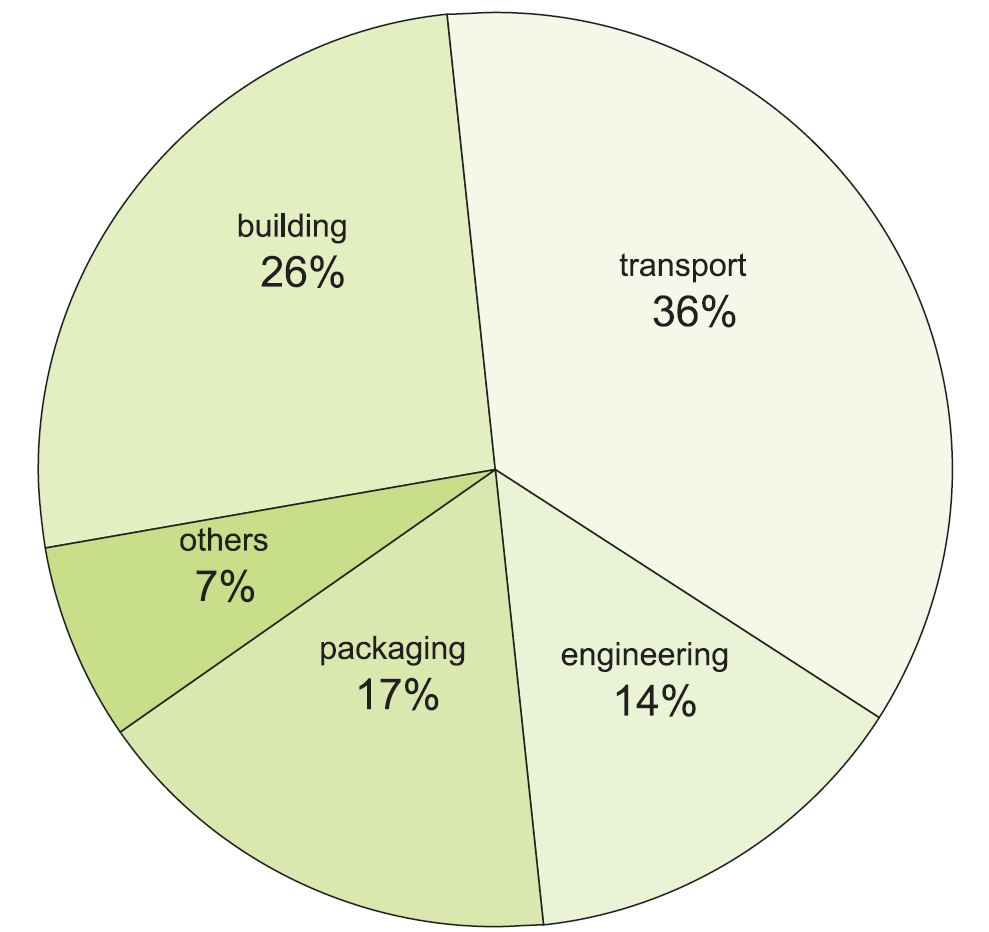
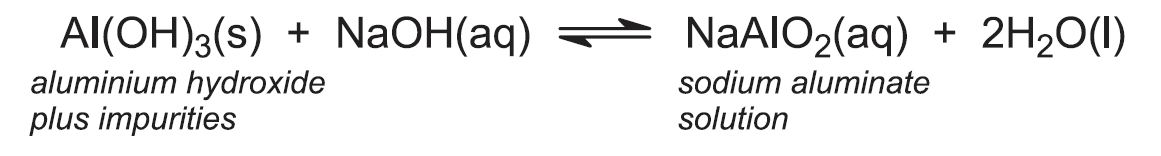Uses of aluminium
Pure aluminium is used principally by the electronics industry for capacitor foil, hard disc drives and conductor tracks on silicon chips. However, when alloyed with small amounts of other metals such as copper, zinc, magnesium and with silicon, aluminium becomes stronger (and can be made even stronger than steel). For example, duralumin (dural) is an alloy of aluminium, copper, manganese and magnesium, aluminium constituting ca 94%.
Aluminium and its alloys are extensively used in transport applications (trains, aeroplanes, ships and cars) where their low density helps reduce fuel consumption and carbon emissions. Bicycles can also benefit from reduced weight and increased strength.
Another important use of the alloys is in packaging, particularly in drinks cans and in foil to protect food. Household uses include saucepans and other cooking utensils, and in buildings the alloys are used extensively in windows, doors and cladding.
Figure 1 Uses of aluminium.
Annual production (Primary aluminium)
These figures are for primary production from the ore bauxite and do not include secondary production from recycled materials.
| World | 58.3 million tonnes |
| China | 32.0 million tonnes |
| Russia | 3.5 million tonnes |
| Canada | 2.9 million tonnes |
| India | 2.4 million tonnes |
| UAE | 2.3 million tonnes |
Data from:
U.S. Geological Survey, Mineral Commodity Summaries, 2016.
Manufacture of aluminium
Primary manufacture involves four processes:
a) extraction of the ore, bauxite
b) purification of bauxite to pure aluminium oxide (alumina)
c) synthesis of cryolite, Na3AIF6 and aluminium fluoride, to be used in the electrolytic reduction process
d) electrolytic reduction of aluminium oxide to aluminium
(a) Extraction of the ore, bauxite
Bauxite is one of the most abundant ores in the world. It is found in particularly large quantities in Jamaica, Brazil, Guinea, China and India. The aluminium occurs in the bauxite ores as the hydroxide Al(OH)3 (gibbsite) and AlO(OH) (boehmite and diaspore).
(b) Purification of bauxite to aluminium oxide
The principal impurities in bauxite are iron(lll) oxide (3 - 25%), silica (1 - 7%) and titanium dioxide (2 - 3%). Powdered bauxite is mixed with approximately 10% sodium hydroxide solution and the resulting mixture heated under pressure (4 atm) at about 420 K. Under these conditions the aluminium hydroxide dissolves as sodium aluminate, but the oxides of iron and titanium remain insoluble. Some silica may also dissolve and so process conditions are chosen to minimise this. The digestion takes about 1-2 hours.
The insoluble residue (known as red mud from the colour of iron oxide) is allowed to settle, washed to recover sodium hydroxide, filtered and then used in land restoration, where the deposited residue is covered with top soil and re-seeded.
The sodium aluminate solution is pumped to large precipitator tanks which may be over 24 m high and have capacities of 1000 m3 or more. The solution is cooled, 'seeded' with aluminium hydroxide crystals and stirred for up to three days, allowing crystallization to occur in a controlled manner so as to achieve the optimum particle size distribution for processing in subsequent stages:
The aluminium hydroxide is separated from sodium hydroxide solution by filtration. A proportion of the aluminium hydroxide is retained for use as seed and the sodium hydroxide solution is recycled. The remaining solid aluminium hydroxide is heated in rotary kilns at about 1300 K to produce aluminium oxide (alumina):
(c) Synthesis of cryolite and aluminium fluoride
The key to the electrolytic reduction of aluminium is the use of cryolite which dissolves the alumina and thus enables it to be electrolysed.
Cryolite is synthesized in several ways. One of these is first to prepare a solution of sodium aluminate from alumina:
Hydrogen fluoride is added to this, and cryolite is then precipitated on addition of sodium carbonate at about 330 K:

Aluminium fluoride is also added to adjust the electrolyte composition and to make up fluoride losses during electrolysis. Aluminium fluoride is produced by the reaction between aluminium hydroxide and fluorosilicic acid:
Fluorosilicic acid is produced as a by-product in the manufacture of hydrogen fluoride and phosphoric acid.
The silica is filtered off and aluminium fluoride crystals are obtained from the filtrate. The silica is then treated with hydrofluoric acid to enable fluorosilicic acid to be recycled:
(d) Electrolytic reduction of aluminium oxide to aluminium
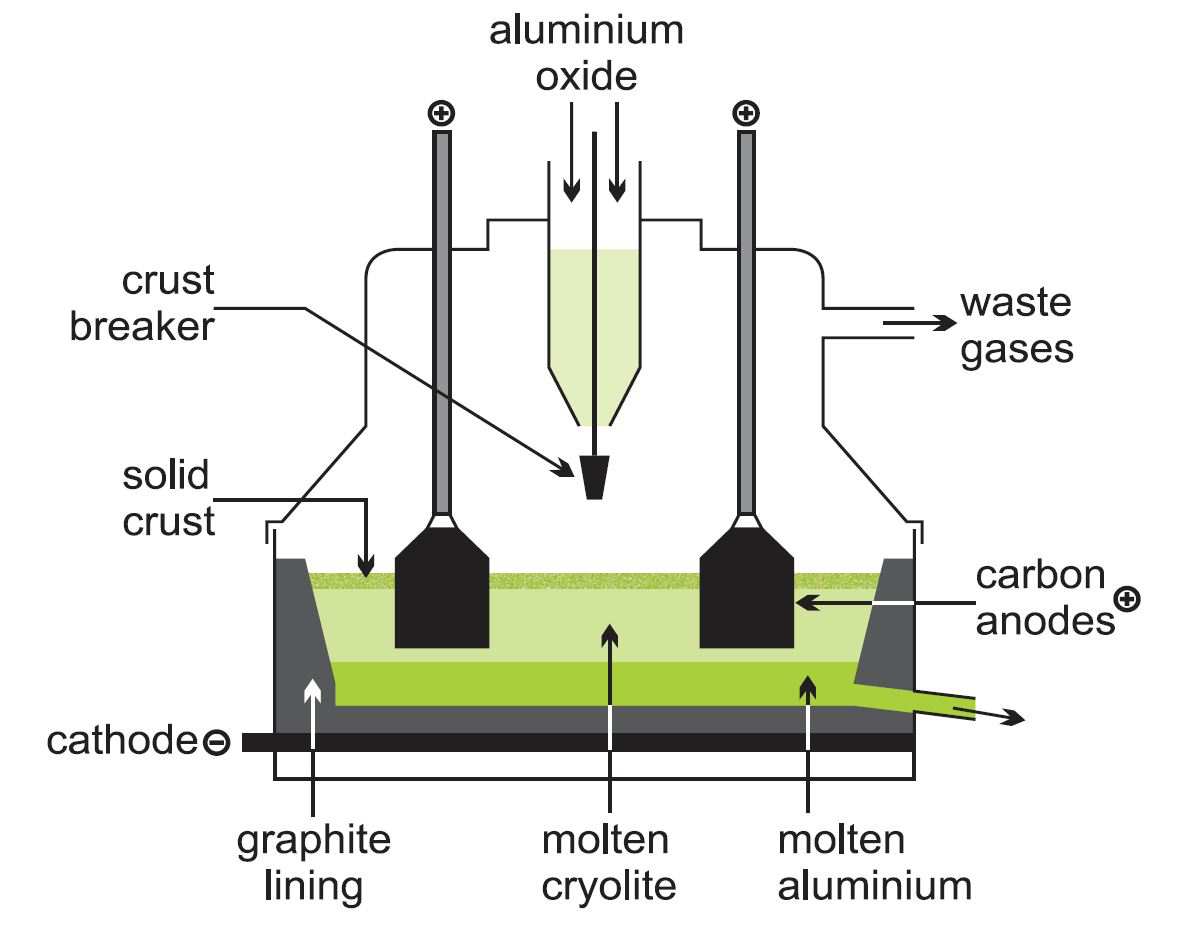
The commercial operation for reducing alumina to aluminium is an electrolytic process invented independently but simultaneously by Paul Heroult in France and Charles Hall in the US in 1886. Huge quantities of electricity are required (10-13 kWh of electricity per 1 kg of aluminium) so aluminium smelters need to be located close to sources of cheap electric power.
The process uses a reduction cell, a shallow open-topped steel box approximately 8 m by 4 m by 1 m deep, the floor of which is lined with graphite (carbon) which acts as the cathode connection. In operation, the box is filled with molten cryolite (at 1200 K) and a small amount (2-5 %) of alumina. The side walls of the box are also lined with carbon. The boxes are designed so that cryolite solidifies in a thin layer on the surface of the electrolyte. Suspended in the electrolyte are several blocks of graphite which form the anode.
|
A typical factory contains about 400 such J cells and produces 300 000 tonnes of metal per year. Associated with it is a factory to fabricate anodes (about 100 000 tonnes of anodes will be required) and a casthouse to produce alloyed metal in a form which can be used by other factories to produce sheet, foil, extrusions, etc.
The exact nature of the electrolyte is not fully known but the overall reactions at the electrodes can be represented as:
Molten aluminium is more dense than cryolite and collects on the cell bottom where it forms the operating cathode. It is normal to operate a cell with a pool of aluminium 10 cm deep, the metal being siphoned off daily to maintain this level. The metal is generally cast as ingots, which are at least 99 % pure with small amounts of iron and silicon being the main impurities.
Alternatively, the metal is kept molten in a furnace to which other elements are added to make alloys, prior to cooling.
Metal of up to 99.999 % purity is produced by further refining.
The process operates continuously with fresh alumina being added regularly to maintain its concentration in the electrolyte at 2-5(w/w)%. Aluminium fluoride is also added to ensure that the fluoride composition is kept constant. The fluoride lowers the melting point of the cryolite-alumina mix, thereby saving power. Some of the fluoride is lost during electrolysis, partly by hydrolysis from moisture in the air which yields hydrogen fluoride:
Figure 3 An electrolytic cell for the manufacture of aluminium.
Carbon is consumed in the anode process, much of it reacting with the liberated oxygen:
The anode blocks must thus be renewed regularly. Typically a cell has twenty anode blocks, one being replaced each day in a twenty day cycle (to replace all of the blocks at once would result in too much cooling of the cell), with the block size being designed to give an operating life of 20-21 days.
Modern cells operate at 150 000 - 350 000 amps and 4.0 - 4.5 volts, each producing 1-2 tonnes of aluminium per day.
The consumption of carbon anodes results in high carbon dioxide emissions from the process. The ideal would be to use inert anodes that are not consumed. Some promising areas of research involve using ceramic materials based on the oxides of tin, antimony and copper. These are conducting, heat resistant and inert, and can be used instead of carbon anodes, significantly reducing carbon dioxide emissions.
Secondary production
Over 50% of the aluminium used to make new products comes from scrap, of which two thirds is 'new scrap'. Aluminium can be easily recycled at low cost (using about 5% of the energy required for primary production) and approximately 60% of European consumption is recycled metal. It has even been estimated that two-thirds of all the aluminium manufactured since commercial production started in1886 is still in use today.
The aluminium scrap, in a steel furnace lined by alumina bricks, is heated from outside the furnace with gas or oil burners. The molten aluminium is then run off and solidified as ingots.
Aluminium can be repeatedly melted and re-used. Recycling 1 kg of aluminium saves up to 8 kg of bauxite ore and 4 kg of other chemical products.
The 'old scrap', used products, are alloys of different compositions so it is better to use the old scrap to remake the same product. An example is new cans made from old cans. The different used products are therefore collected and sorted before being remelted. To make recycling even more efficient, the gases produced when burning off coatings used for labelling can be used as fuel for melting the scrap metal.
Date last amended: 24th September 2016