Zinc

Uses of zinc
Zinc is used as coating to protect iron and steel from corroding in the atmosphere, water and soil. This is because zinc reacts preferentially to iron in most environments to form protective layers of oxide, carbonate or other zinc reaction products that are resistant to subsequent corrosion by the atmosphere. Even if the coating is scratched it continues to corrode preferentially and protect the iron. Zinc is the sacrificial metal.
There are various methods of coating iron and steel with zinc. One is to dip the article into a bath of molten zinc, a process known as hot dip galvanizing.
Steel is often galvanized by uncoiling rolled steel sheet and passing it through a bath of molten zinc. It is then used for many purposes, particularly in the manufacture of white goods (refrigerators and washing machines) and car bodies.
Steel articles already fabricated, such as electricity distribution poles, highway guard rails and irrigation pipes, can be completely coated by cleaning and then immersing in a zinc bath, a process termed general galvanizing. Fasteners can also be immersed and then centrifuged whilst the coating is still liquid to produced thin coatings. Alternatively, less durable coatings on fasteners and sheet can be produced by electroplating.
Another method, used for small articles such as nails and screws, is to heat them in a rotating drum with zinc dust at ca 650 K. This is known as sheradizing.
Very large objects such as bridges can be protected after installation by being sprayed with liquid zinc.
Zinc alloy anodes are used to protect marine oilrigs and ships. They are attached to the steel and corrode in preference to the steel. They are replaced when they have been completely corroded.
Zinc-aluminium alloys are used in die-casting, a generic term for casting in a mould using a pressure injection technique. It results in a high-precision casting of moderate strength that is capable of being highly finished. A wide range of products is made in this way, from die-cast toys to components for engines.
|
|
 |
|
* zinc semis are sheets, rods and plates of zinc metal Figure 1 Uses of zinc (2014) Data from: statista.com |
Figure 2 Part of the Jewish Museum in Berlin. The architect, Daniel Liebskind, used zinc, alloyed with titanium, as an outer surface of this remarkable building. By kind permission of Jewish Museum Berlin, picture Jens Ziehe. |
Zinc is used in the ordinary dry cell battery (the preferred type for devices that do not need much power, such as remote controls). The zinc is used as the outer casing and the anode at one and the same time. The cathode is a mixture of carbon and manganese(IV) oxide and the electrolyte is a mixture of ammonium and zinc chlorides.
Other zinc alloys (mainly rolled) are frequently used for semi-manufactured products such as coins, the current-carrying portion of small electrical fuses, and anode ribbon for buried pipelines.
Brass, an alloy containing primarily copper and zinc, is used to make both decorative and functional products like door handles, marine fittings, plumbing components and screw fixings.
Annual production of zinc
| World | 13.4 million tonnes |
| China | 4.9 million tonnes |
| Australia | 1.6 million tonnes |
| Peru | 1.4 million tonnes |
| U.S. | 0.8 million tonnes |
| India | 0.8 million tonnes |
Data from:
U.S. Geological Survey, Mineral Commodity Summaries, 2016.
China now accounts for about 37% of the world's production of the metal.
Manufacture of zinc
Nearly all zinc is obtained from sulfide ores, which also usually contain lead, cadmium and other metals such as iron and silver. The most commonly occurring ores are sphalerite, also known as zinc blende (ZnS), and another variety of sphalerite called marmatite which contains significant quantities of iron sulfides.
Sulfide ores are widely distributed throughout the world. The major deposits are found mainly in North and South America (Canada, US, Mexico, Peru, Bolivia), Australia, Japan and China. There are also significant deposits in South Africa, Iran, Spain, Scandinavia, Spain, Macedonia, Russia and Germany.
Figure 3 Drilling zinc and lead ore in the Black Mountain Mine in the Northern Cape, South Africa.
By kind permission of Anglo American.
There are two main processes: the electrolytic process and the thermal process. Over 90% of the world's production comes from the electrolytic process.
The electrolytic process
The process has four stages:
a) concentration of the ore
b) roasting of the ore in air
c) conversion of zinc oxide to zinc sulfate
d) electrolysis of zinc sulfate solution
(a) Concentration of the ore
The ore is mined, crushed, ball-milled and then concentrated by froth flotation. This removes unwanted components, including the lead compounds and waste rock.
(b) Roasting of the ore in air
The ore roasting usually takes place in a fluidised bed furnace at around 1300 K, with air being blown in at the bottom. The most important reaction is the conversion of zinc sulfide to zinc oxide:
However, any iron sulfide present in the ore will be converted to iron(III) oxide, which reacts with zinc oxide to form zinc ferrite:
In the simple leaching process, this zinc cannot be easily recovered and so ores with low iron content are preferable.
The sulfur dioxide is often converted to sulfuric acid in a plant adjacent to the smelter.
(c) Conversion of zinc oxide to zinc sulfate
The crude zinc oxide is leached with spent electrolyte, which is sufficiently rich in sulfuric acid to dissolve the oxide and restore the concentration of the zinc sulfate in the electrolyte solution (Figure 4).
The main reaction taking place is:
Figure 4 Leaching of zinc oxide.
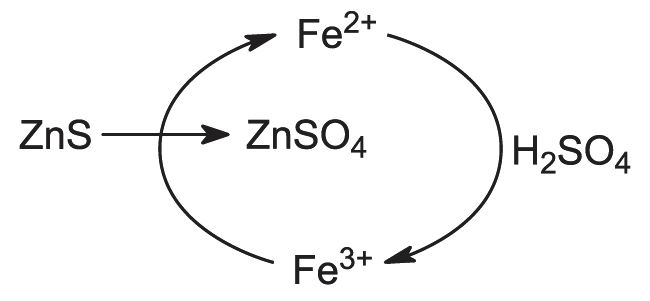
As mentioned above, some of the zinc oxide is present with iron(III) oxide in the form of zinc ferrite. Several variations on the leaching process are used to separate zinc from impurities. Most of these use hot acid conditions to produce a mixture of zinc and iron(III) sulfates, followed by removal of the iron(III) sulfate.
One method (Figure 5) precipitates the iron as a 'jarosite' (jarosites are compounds based on iron(III) sulfate, and found in some mineral deposits).
Figure 5 Recovery of zinc oxide from zinc ferrite.
After the hot acid leaching, the precipitation of jarosite takes place using ammonium or sodium compounds, and the liquid product is then passed to the milder leaching stage. The reaction also produces sulfuric acid, and roasted ore may be added at the jarosite stage to help control acidity:

The mixture containing zinc sulfate is then filtered to remove suspended solid matter, and the solution is treated with zinc dust to precipitate the less electropositive metals. For example, cadmium is a valuable by-product of the operation:
Alternative processes for producing zinc sulfate
Direct Leaching
Several methods have now been developed that dispense with the roasting stage, obtaining zinc sulfate direct from concentrated zinc sulfide ore. They generally use much more extreme conditions, and are suitable for lower-grade ores. One process developed in Canada, and capable of recovering 99% of the zinc in the ore, uses pressures in excess of 10 atmospheres and a temperature of ca 420 K. The presence of iron in the ore concentrate is important in this method, as it is in part responsible for the conversion of zinc sulfide to zinc sulfate.
Iron(ll) sulfate Is oxidized to iron(lll) sulfate by the hot, acid conditions.
This then oxidizes zinc sulfide to zinc sulfate, and is reduced back to iron(ll) sulfate in the process.
Zinc sulfide also reacts with sulfuric acid under these conditions:
As shown in the equation, the process also produces sulfur as the element, and this can be used to make the sulfuric acid required.
Bioleaching
Very high zinc extraction rates (up to 99%) from low-grade ores (as low as only 5% zinc) can also be achieved using bacterial action. The bacteria used thrive at temperatures up to ca 320 K, and produce weak zinc solutions that are concentrated for electrolysis using solvent extraction.
(d) Electrolysis of zinc sulfate solution
The last stage entails the purification of zinc by electrolysis of a solution of zinc sulfate.
Figure 6 The electrolytic cells have aluminium cathodes on which zinc is deposited and is then scraped off.
By kind permission of Anglo American.
Zinc is liberated preferentially at the cathodes. Every 24 to 72 hours zinc is stripped off the electrodes, melted and cast into ingots. The metal is at least 99.96% pure.
At the anodes, oxygen is evolved. The sulfuric acid is regenerated and recycled and mixed with fresh ore:
It is possible to make very high purity zinc (99.995% pure) by adjusting electrolysis conditions such as temperature and current density. Metal of this purity is required for diecasting alloys containing aluminium, magnesium and copper.
The thermal process
The current thermal process uses the Imperial Smelting Furnace, ISF, which was invented and developed at Avonmouth, Bristol. Although formerly prominent, its relatively high energy and emmisions costs led to it being completely superseded in Europe by the electrolytic process, although ISFs still operate on other continents. It is capable of simultaneously producing zinc and lead from sinter (agglomerated oxides). Sinter is produced by roasting a mixture of zinc and lead concentrates, fluxing agents (sand and lime), and secondary materials.
In the ISF process, a blast furnace is charged with the sinter and hot coke. Hot air (I200 - I350 K) is blown into the furnace through tubes called tuyeres. The reactions occurring in the furnace may be summarised as:
The melting and boiling points of the two metals are:
| Lead | Zinc | |
|---|---|---|
| Melting point/K | 600 | 693 |
| Boiling point/K | 2024 | 1181 |
Under the conditions in the furnace zinc is a vapour (gas), whereas lead is produced as a liquid by a similar series of reactions.
The other components of sinter such as silica (SiO2), lime (CaO), alumina (Al2O3) and iron oxide (Fe2O3/FeO), form a molten slag of silicates. This is tapped from the hearth of the furnace simultaneously with the lead, and then separated in a vessel called a forehearth. Lead is on the bottom layer and is cast into 2 to 4 tonne blocks. It is conveyed as the liquid metal and is converted into refined lead. Molten slag (1300-1550 K) is granulated by a water jet and set aside.
The zinc vapour is carried into the condenser in a stream of carbon monoxide and carbon dioxide gases (at about 1300 K) and condensed by adsorption in a spray of liquid lead.
The lead splash condenser sprays lead using a series of rotors. The resultant lead/zinc mixture is pumped from the condenser (at about 830 K) and cooled using water-cooled immersion coolers (to about 710 K). The lead/zinc mixture passes into a separation bath where the zinc floats to the surface and the lead returns to the condenser via an underflow. Zinc passes over an overflow weir and is tapped off and conveyed to the zinc reinery or casting area.
ISF zinc from the furnace contains about 1-1.3% lead and may be reined by distillation to produce better than 99.95% purity zinc.
Metallic cadmium and other valuable metals may also be recovered in the reining process.
Secondary production
Over 35% of the zinc used annually is from recycled metal. Much of this comes from zinc-coated steel which, for example, has been used for rooing.
This is placed in the Electric Arc Furnace being used to recycle the steel. Zinc is relatively volatile and leaves the furnace with other gases. It is collected on cooling as zinc dust. This is heated in air to form zinc oxide which, in turn, is treated with sulfuric acid to form zinc sulfate and from which pure zinc is obtained, as described above.
Date last amended: 5th October 2016