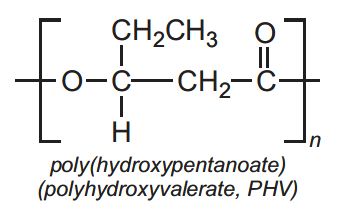As with so many technological advances, plastics bring not just enormous benefits to society but also grave threats if misused. On the one hand they allow us to make goods that would otherwise be impossible to fabricate economically, goods that we rely on in everyday life. On the other hand, plastics have two great environmental handicaps. First, the vast majority are made from non-renewable fossil fuels, oil and natural gas. Secondly, their desirable virtues of strength, inertness to chemicals, microorganisms and light also make them difficult to dispose of in an eco-friendly way. With natural polymers, specific enzymes have developed over billions of years to aid rapid degradation; for most of the plastics developed over the last 80 years, no such enzymes exist.
There are already several solutions to the problems of both manufacturing and disposing of plastics, but these are not always mutually compatible. Making a plastic from a renewable resource does not necessarily mean that its disposal will be eco-friendly. For example, poly(ethene) could (and a small proportion soon will) be made from biomass via ethanol and ethene but it is still very difficult to degrade. Conversely, polycaprolactone is a biodegradable plastic but is produced via cyclohexanone, itself derived from benzene (whose manufacture is based on oil).
In the following sections these types of degradable plastics are discussed:
- plastics produced from renewable resources
- plastics produced from fossil fuels
- plastics that are photodegradable
Degradable plastics from renewable resources
The poly(hydroxyalkanoates), PHAs, are now much used. They are made from renewable materials and are readily degradable.
PHAs are polyesters which are produced by bacteria grown on certain substrates from which nutrients such as nitrogen are withheld. The bacteria are encouraged to produce an energy store for later use, similar to the way that plants produce starches. Considerable work is being devoted to producing genetically-engineered e.coli to grow PHAs in this manner.
Amongst these polyesters, poly(hydroxybutanoate), PHB, is the best known. It is produced by fermentation of glucose solutions (produced from starch by hydrolysis) to which propanoic acid has been added. Sources of nitrogen as a nutrient are withheld. The bacterium is Ralstonia metallidurans.
The polymer has physical properties similar to those of poly(propene). Unlike many other bioplastics, it is water-insoluble and resistant to UV radiation. However, it is readily hydrolyzed by acids and thus can be easily degraded.
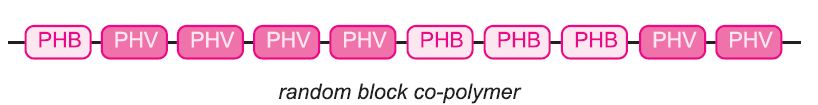
A well known co-polymer is Biopol. It is a random co-polymer with blocks of PHB and blocks of poly(hydroxypentanoate), (often known as polyhydroxyvalerate, PHV).
Like other PHAs, the film is used to wrap foods, coatings for paper and cardboard, kitchen ware (cups, saucers, plates), and many medical uses, including sutures, gauzes and coatings for medicines.,/p>
Another degradable polyester is poly(2-hydroxypropanoic acid) (polylactic acid, PLA), produced from 2-hydroxypropanoic acid (lactic acid).
The lactic acid, traditionally made from ethanal (acetaldehyde) and hydrogen cyanide, is now being increasingly produced by an alternative method, the fermentation of sugars from maize (corn syrup) and cane sugar (molasses) using the lactobacillus bacterium.
However, the acid cannot be polymerized directly as the water produced prevents the polymerization process proceeding to high enough molecular masses. The acid is therefore first dimerized by heating the acids strongly. The dimer undergoes ring opening polymerization using tin octanoate as a catalyst. This method avoids the formation of water during polymerization:
|
Figure 1 This cup is made from PLA and another polymer, a polyester made from butane-1,4-diol reacting with two acids, benzene-1,4-dicarboxylic acid (terephthalic acid) and hexanedioic acid (adipic acid). The co-polymer can also be produced as a shrink film for packaging and as a foam for packaging fast foods. Over 75% of these polymers are derived from renewable sources and are readily degradable. |
 |
 |
This may appear to be the more ecologically sound of the two processes but it does have the drawback of being energy intensive because of the purification procedures that have to be used. Further, ethanal, the traditional precursor of the acid could be produced in the future from ethanol, produced from biomass.
The properties of PLA such as its strength can be improved using additives without reducing its very good optical properties (transparency) and it is used for food packaging, bottles, cups and dishes and grocery bags. It is also readily composted.
Co-polymers of PLA with poly(hydroxyethanoic acid) (polyglycolic acid), PGA) have found considerable use in surgery. Threads of the co-polymer, which is a block co-polymer are used for stitching internal wounds and are degraded back to their parent monomers within 90 days after surgery. The acids are biocompatible with the body.
| Figure 2 Some polymers based on starch are derived from renewable resources and are readily degradable. The starch used is from corn (maize). This contains a high proportion of amylose, which consists of linear chains of glucose units. The starch is treated with epoxypropane (propylene oxide) which reacts with some of the hydroxy! groups in the amylose. The resulting polymer can be used in place of polymers such as poly(propene) and poly(chloroethene), PVC. It is being increasingly used for packaging, for example as bags and as shown here in packaging for biscuits and chocolates. By kind permission of DuPont. |
 |
 |
Figure 3 A demonstration of the ease by which a polymer derived from starch can be degraded. The polymer is also composted readily by microorganisms in the soil. By kind permission of DuPont. |
Degradable plastics from fossil fuels.
These polymers are synthetic biodegradable polymers, that is polymers which are not made by biological processes, but which will still degrade.
One type are the synthetic polymers such as poly(ethene) which have starch granules encapsulated in the structures, the amount varying from 5-50 %. These granules are degraded by bacteria and the tiny fragments of poly(ethene) produced degrade at a faster rate than poly(ethene) without the starch, although more slowly than biopolymers. Also, their production wastes valuable oil unless the poly(ethene) has been derived from a biorefinery which is still restricted to a few factories in Brazil.
Other synthetic polymers which have been treated with starch granules include poly(propene) and poly(phenylethene).
There is another group of polymers derived from fossil fuels which degrade readily in composts, an example being poly(caprolactone), produced via benzene and cyclohexanone.
Like PLA, it hydrolyzes slowly in acid conditions and is used for stitching internal wounds and for drug delivery, where the drug is coated with the polymer. The coating dissolves slowly allowing the medicine to be delivered over a measured period of time. Another use is in making models. The polymer, a white solid, can be warmed, becoming pliable and the resulting 'putty' can be modelled. The polymer is sometimes blended (melted and extruded together) with PHB.
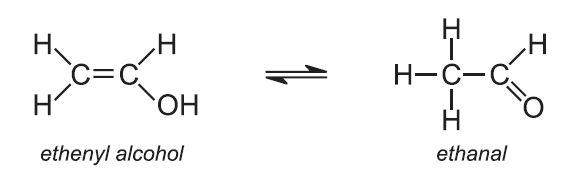
Another important example of a fast degrading polymer derived from a petrochemical source is poly(ethenyl alcohol), known better by its older, unsystematic name, polyvinyl alcohol, PVA. Over 1 million tonnes are made annually worldwide.
PVA cannot be made from its parent monomer, ethenyl alcohol (vinyl alcohol), as this would immediately form its more stable tautomer, ethanal:
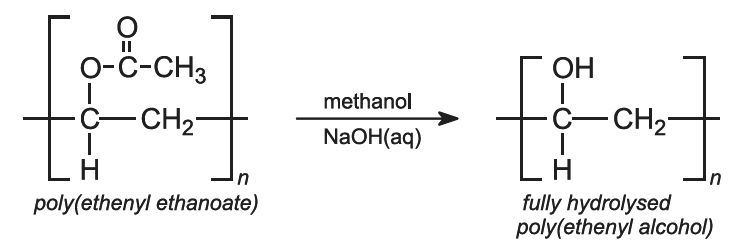
Instead PVA is made from the polymer poly(ethenyl ethanoate), usually known as polyvinyl acetate.
The polymer is treated with methanol and an aqueous solution of sodium hydroxide. For some uses, all the ethanoate groups are hydrolyzed to form hydroxyl groups (fully hydrolyzed PVA):
However, for other uses, only partially hydrolyzed PVA is used.
PVA is a readily degradable water-soluble polymer and among its uses are as an adhesive (paper, fabrics, leather) and for coating paper and paperboard. It is also used as a thickener in a wide range of liquids including paints, glues and hairsprays. However its main use is in making polyvinyl butyral, PVB. Partially hydrolyzed PVA, containing 1-3 % ethanoate groups, reacts with butanal to form PVB which has very good optical clarity, is tough and bonds strongly to glass. However it will be susceptible to hydrolysis.
PVB is used extensively as the middle layer in laminated (safety) glass used, for example, in cars.
Photodegradable polymers
These are polymers whose disintegration is initiated by sunlight. Some have functional groups (such as carbonyl groups) with bonds which absorb UV radiation. They trap enough energy for nearby bonds to be ruptured, thereby breaking the polymer chain into smaller fragments. These smaller fragments degrade more quickly than longer chains. Nonetheless they suffer from the same disadvantages as synthetic biodegradable polymers in that they are derived from materials which have been obtained from fossil fuels.
Another strategy is to add a compound to the plastic, known as a prodegradant additive. Some are known as Totally Degradable Plastic Additives, TDPAs. They are added to conventional polymers such as poly(ethene) and poly(propene) and used, for example, in shopping bags.
Examples of additives include cobalt(III) and manganese(III) octadecanoates (stearates), usually at concentrations of 1-2%. Added at the extrusion stage, they promote free radical attack on the polymer and thus initiate the degradation and as above, cause chain scission to form smaller fragments at which stage bacterial degradation occurs. The polymers are degraded within a few months in a conventional compost facility, and have better mechanical properties than the starch-filled polymers.
The main use for these polymers is in shopping bags and food wrapping.
Date last amended: 18th March 2013