Uses of poly(propene) (polypropylene)
Poly(propene) is processed into film, for packaging and into fibres for carpets and clothing. It is also used for injection moulded articles ranging from car bumpers to washing up bowls, and can be extruded into pipe (Figure 1).
Figure 1 Uses of poly(propene).
Materials suitable for a much wider range of applications can be made by compounding poly(propene) with, for example, fillers and pigments, and elastomers.
Poly(propene) has remarkable properties, making it suitable to replace glass, metals, cartons and other polymers. These properties include:
- low density (weight saving)
- high stiffness
- heat resistance
- chemical inertness
- steam barrier properties (food protection)
- good transparency
- good impact/rigidity balance
- stretchability (film and fibre applications)
- good hinge property (for example where a lid and box are made together, for DVD boxes)
- high gloss (appearance)
- easy to weld (design)
- recyclabililty
The majority (ca 60% of the total produced) of poly(propene) is produced as a homopolymer. Co-polymers are discussed below.
Poly(propene) is one of the lightest thermoplastics (density 0.905 g cm-3). It has a melting point of 440 K and a crystallinity of ca 50-60%. The polymer, unlike poly(ethene), is transparent.
Structure of the polymer
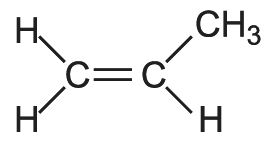
The propene molecule is asymmetrical,
and, when polymerized, can form three basic chain structures dependent on the position of the methyl groups: two are stereoregular (isotactic and syndiotactic) and the third does not have a regular structure and is termed atactic as shown diagrammatically below:
Figure 2 Molecular structures of poly(propene).
The 'one handed' structure of isotactic poly(propene) causes the molecules to form helices. This regular form permits the molecules to crystallize to a hard, relatively rigid material, which, in its pure form, melts at 440 K.
The syndiotactic polymer, because of its regular structure, is also crystalline.
Atactic chains are completely random in structure and consequently they do not crystallize. High molecular mass atactic poly(propene) is a rubber-like material.
Commercial poly(propene) is a predominantly isotactic polymer containing 1-5% by mass of atactic material.
Annual production of poly(propene) (polypropylene)
| World | 52.2 million tonnes |
| Europe | 13.1 million tonnes |
| Russia | 0.64 million tonnes1 |
Data from:
1. Federal State Statistics Service: Russian Federation 2011
Manufacture of poly(propene) (polypropylene)
Poly(propene) is produced from propene. Propene is produced in large quantities from gas oil, naphtha, ethane and propane.
Parallel to this, there are several methods being developed to produce bio-based poly(propene) (bio-based polypropylene) via bio-based-propene.
(a) Using a Ziegler-Natta catalyst
Ziegler-Natta catalysts are used in the polymerization process. These are produced by interaction of titanium(IV) chloride and an aluminium alkyl, such as triethyl aluminium.
Two main processes are used for making the polymer with these catalysts, although the slurry method is used as well.
(i) The bulk process
Polymerization takes place in liquid propene, in the absence of a solvent at a temperature of 340-360 K and pressures of 30-40 atm (to keep the propene as a liquid). After polymerization, solid polymer particles are separated from liquid propene, which is then recycled.
The use of liquid propene as a solvent for the polymer as it is formed means that there is no need to use hydrocarbons such as the C4-C8 alkanes which are used in the parallel manufacture of poly(ethene).
|
(ii) The gas phase process
A mixture of propene and hydrogen is passed over a bed containing the Ziegler-Natta catalyst at temperatures of 320-360 K and a pressure of 8-35 atm.
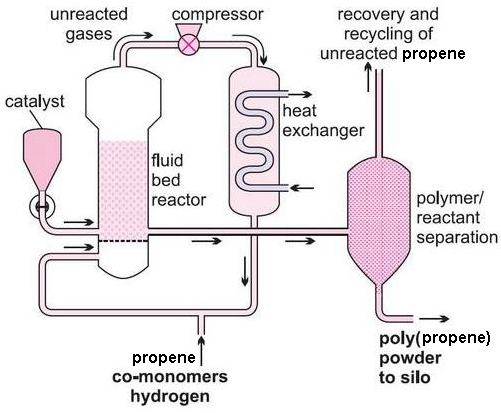
Figure 4 Low pressure gas-phase process
The polymer is separated from the gaseous propene and hydrogen using cyclones and the unreacted gas is recycled.
Both processes can be operated continuously and use 'stereospecific' Ziegler-Natta catalysts to effect the polymerization. The catalyst remains in the product and needs to be destroyed using water or alcohols, before the polymer is converted into pellets.
Both bulk and gas phase processes have virtually eliminated gaseous and aqueous effluents by the use of high activity catalysts, resulting in low residues in the final polymer.
(b) Using a metallocene as catalyst
Metallocenes are being increasingly used as catalysts for the production of poly(ethene) (mLLDPE) and poly(propene).
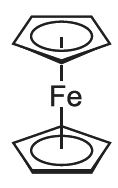
Metallocenes are strictly defined as molecules which have a transition metal atom bonded between two cyclopentadienyl ligands which are in parallel planes. Ferrocene is a particularly well known example:
However, the term is now used more widely to include other ligands related to cyclopentadienyl. One such metallocene is based on zirconium:
Zirconium has an oxidation state of 4 and is bonded to two indenyl ligands (a cyclopentadienyl ligand fused to a benzene ring). They are joined by two CH2 groups. In conjunction with an organoaluminium compound, it acts as a catalyst for the polymerization of alkenes such as ethene and propene. The specific orientation of the zirconium compound means that each propene molecule, for example, as it adds on to the growing polymer chain is in the same orientation and an isotactic polymer is produced.
When a different zirconium compound is used,
the syndiotactic form of poly(propene) is produced. This is the only way of making syndiotactic poly(propene) commercially.
As with the Ziegler-Natta catalysts, the bulk or gas phase (described above) can be used. Alternatively the slurry process is used.
Poly(propenes) made in this way, mPP, are used in particular to make non-woven fibres and heat-seal films.
Metallocenes also catalyse the production of co-polymers of propene and ethene.
Co-polymers
There are two main types of co-polymer. The simplest are the random co-polymers, produced by polymerizing together ethene and propene. Ethene units, usually up to 6% by mass, are incorporated randomly in the poly(propene) chains (Figure 5).
Figure 5 Illustrating an alternating co-polymer formed from propene and a small amount of ethene.
The crystallinity and melting point are reduced and the products are more flexible and are optically much clearer. Major uses for these random co-polymers are for medical products (pouches, vials and other containers) and packaging (for example, bottles, CD and video boxes).
Many other co-polymers of ethene and propene, with higher alkenes such as hexene, are being developed which will produce polymers similar to LLDPE but which have better mechanical and optical properties.
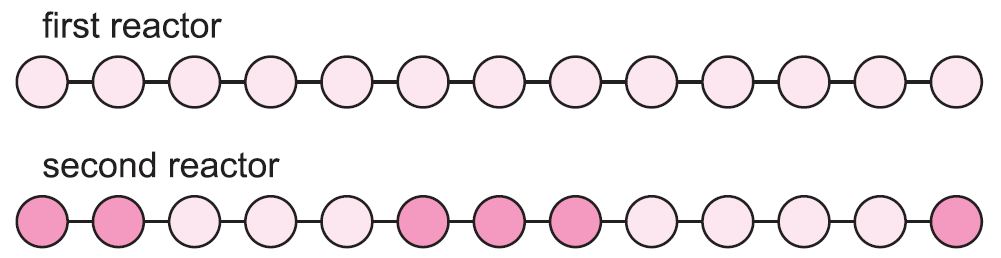
The second type of co-polymers is the so-called 'block' co-polymers. These are made by following the poly(propene) homopolymerization with a further, separate stage, in which ethene and propene are co-polymerized in the gas phase. Thus these two processes are in series (Figure 6).
Figure 6 lllustrating the homopolymer and the block co-polymer formed from propene and ethene.
The products of these two processes form a composite in which nodules of the block co-polymer are distributed with the homopolymer (Figure 7).
Figure 7 The propene-ethene block co-polymer nodules dissipate impact energy and prevent cracking.
The ethene content of the block co-polymer is larger (between 5 and 15%) than used in randomly alternating co-polymers. It has rubber-like properties and is tougher and less brittle than the random co-polymer. Consequently, the composite is particularly useful in making crates, pipes, furniture and toys, where toughness is required.
When ethene, propene and a third monomer, a diene, are polymerized, a rubber is formed, known as EPDM (Ethene, Propene, Diene, polyMethylene. The ethene and propene molecules polymerize to form very long molecular chains, with several thousand monomer molecules in a chain.
The polymerization is usually effected in solution using a Zeigler-Natta catalyst but more recently, metallocenes have been used very successfully. Usually the ethene content is about 60% and that of the diene varies between 2 and 7%. The polymer chain has the structure,
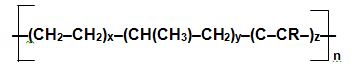
where R contains one carbon-carbon double bond.
As can be seen from the formula, it is a block co-polymer.
As the diene (usually ENB ethylidene norbornene) has two double bonds, one is used in the chain and the other is used to form a three dimensional structure. The reactive sites are pendant (not part of the backbone of the chain) and are joined together in the next part of the process when the polymer is heated with sulfur, the process used to vulcanize rubber. The two dimensional structure shown above becomes three dimensional.
Date last amended: 21st August 2016