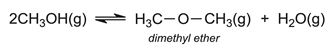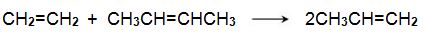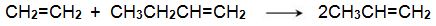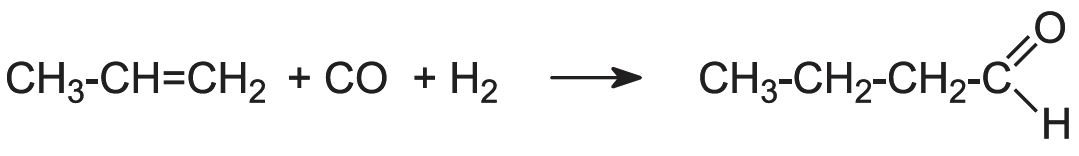
Uses of propene (propylene)
Figure 1 The uses of propene1.
1. Data for 2015, J S Plotkin The Propylene Quandary, American Chemical Society, 2016
The principal uses of propene are to produce:
- poly(propene) (polypropylene)
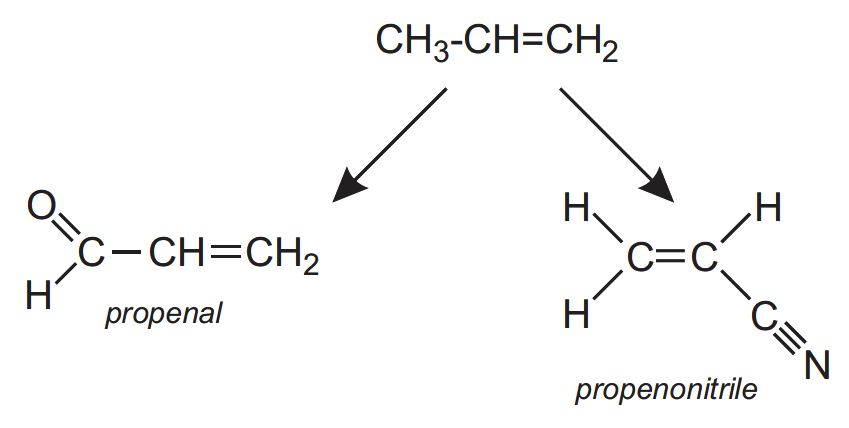
- propenal (acrolein) which is oxidized to propenoic acid (acrylic acid) which, in turn, is used to make acrylic polymers
- propenonitrile (acrylonitrile) which is the monomer for poly(propenonitrile)
- cumene ((1-methylethyl)benzene or isopropylbenzene) which is then used to make phenol and propanone (acetone)
- epoxypropane (propylene oxide) which is used to make diols for the manufacture of polyurethanes and solvent
- butanal (butrylaldehyde) and hence butanol, used as a solvent for surface coatings
The data given in Figure 1 are for global production. However, the data vary from country to country. For example, the proportion used to make poly(propene) varies from only 55% in North America and 57% in Europe to 90% in the Middle East. The global proportion used to make epoxypropane (propene oxide) is 7% but 15% of the propene in Europe is used to make the epoxide2.
2. Data for 2015, Petrochemicals Europe
Annual production of propene (propylene)
| World | 94 million tonnes1 |
| Asia Pacific | 27 million tonnes2 |
| Europe | 15 million tonnes2,4 |
| US | 13 million tonnes3 |
| Middle East | 7.5 million tonnes2 |
Data from:
1. Data for 2015, J S Plotkin, The Propylene Quandary, American Chemical Society, 2016
2. Estimated data for 2014, Global Petrochemical Overview: Changing Olefins Markets, Nexant and ChemVision, 2014
3. 2015 Guide to Business of Chemistry, American Chemical Council, 2016
4. Data for 2015, Petrochemicals Europe
Manufacture of propene (propylene)
For many years, the overwhelming proportion of propene has been manufactured by two processes
- steam cracking of naphtha
- catalytic cracking of gas oil
Both processes also produce many other products, for example, ethene in large amounts.
However, the amount of propene needed compared with that of ethene has increased and much research and development has concentrated on finding methods of producing propene without ethene. These are known as OPP processes (On-purpose propene). There are now three being used in significant amounts.
(i) Catalytic cracking of propane
Increasingly, propane is being catalytically cracked to form propene, using the same cat cracker as that used to crack gas oil:
![]()
Propane is being obtained in increasingly large amounts in the US from shale gas extracted by fracking.
(ii) The MTO (Methanol To Olefins) Process
Another process that has been developed is via methanol (produced from biomass via synthesis gas), the MTP (Methanol To Propene) process which is an example of the MTO (Methanol To Olefins) process. (Olefin is the older name for the homologous series, alkenes). Methanol can be converted into high purity ethene and propene via dimethyl ether. Methanol vapour is passed over alumina at ca 600 K. An equilibrium mixture of methanol, dimethyl ether and steam is produced, containing about 25% methanol:
This mixture of gases is then passed over a bed of a zeolite in a form that encourages high selectivity towards alkenes with numbers of carbon atoms from 2 to 8. However, by using a zeolite treated with acid, almost all the alkene produced is propene. The propene is purified by cooling it to a liquid and then subjecting the liquid to fractional distillation.
This is a similar process to that used to make hydrocarbons used in gasoline, the MTG (Methanol to Gasoline) process.
(iii) The reaction between ethene and butenes
Another promising method is one from ethene and butene:
Bio-based ethene can be obtained by dehydration of bioethanol using a silica/alumina or alumina catalyst. The butenes (but-1-ene and but-2-ene) can be produced by either dehydration of biobutanol or by dimerization of bioethene).
The dimerization of ethene to but-1-ene is carried out by passing heated ethene over a zeolite impregnated with a transition metal complex. A variety of complexes of rhodium, titanium and other metals are used:
A mixture of ethene and butene is then heated and passed over a solid catalyst based on organic compounds of molybdenum(IV) and tungsten(IV) (the Schrock catalysts) and organo-ruthenium (II) compounds (the Grubbs’ catalysts), in a fixed-bed reactor:
Small amounts of coke are deposited on the catalyst and are removed from time to time by passing heated air through the reactor.
In 2010, steam cracking accounted for ca 56% and catalytic cracking of gas oil for 37% of the global production. Much of the rest was made from the production of oil from coal and from the cracking of gas oil under vacuum. However, the new processes are being increasingly used and now account for some 15% of the propene produced and, will, it is expected, account in 2020 for about 25% of the propene being produced, with steam cracking accounting for about 42% and cracking about 33%1.
1. J S Plotkin The Propylene Quandary, American Chemical Society, 2016 Data for 2015
Another method of producing propene is via syngas and ethanol. Synthesis gas (carbon monoxide and hydrogen) is used to convert bioethanol to propan-1-ol:
The reaction is catalysed by a ruthenium-cobalt complex salt. A molybdenum-based catalyst is also being used as it is more resistant to poisoning by sulfur-containing impurities in the feedstock.
Subsequently, propan-1-ol is dehydrated to propene:
Note on the chemistry of ethene (ethylene) and propene (propylene)
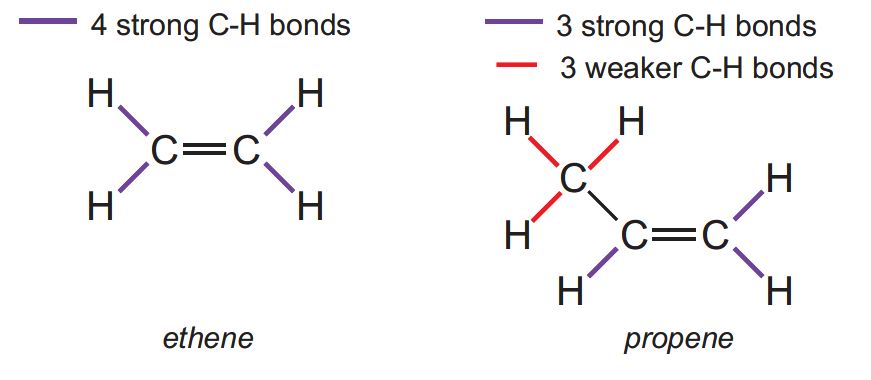
All the C-H bonds in ethene are very strong and thus the majority of its reactions involve addition to the double bond.
Similarly, propene has 3 strong C-H bonds and undergoes addition reactions (for example, polymerization to poly(propene) and propene to epoxypropane).
However, the C-H bonds in the methyl group are much weaker and propene has many reactions in which the double bond is preserved and the methyl group undergoes substitution reactions, for example:
Manfacture of epoxypropane (propylene oxide)
Epoxypropane is manufactured in three main ways:
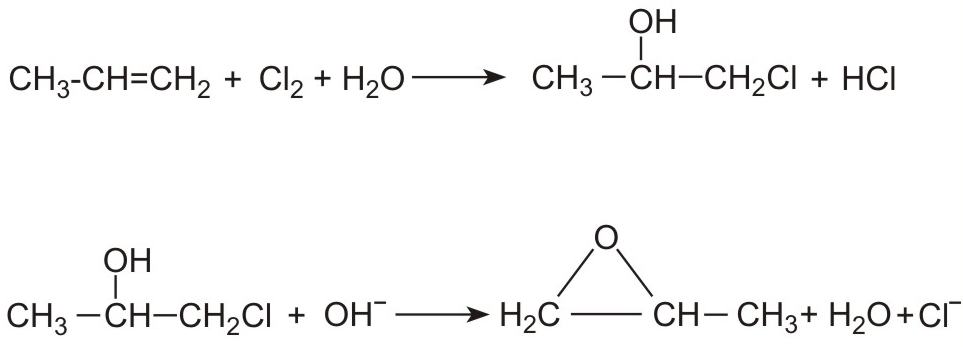
(i) By reacting propene with an aqueous solution of chlorine to form a mixture of 1-chloropropan-2-ol (90%) and 2-chloropropan-1-ol (10%). Epoxypropane (propylene oxide) forms on addition of a solution of either sodium hydroxide or calcium hydroxide. For example:
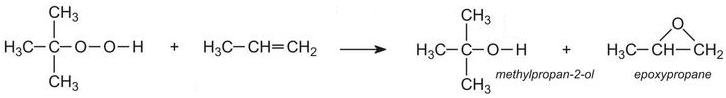
(ii) By reacting propene with a hydroperoxide such 1,1-dimethylethyl hydroperoxide.Propene is passed into liquid 1,1-dimethylethyl hydroperoxide under pressure at about 400 K with a soluble molybdenum salt as catalyst:
(iii) By reacting propene with hydrogen peroxide. New plants have been built, adjacent to the plants producing propene, to manufacture large quantities of hydrogen peroxide. The peroxide reacts directly with propene:
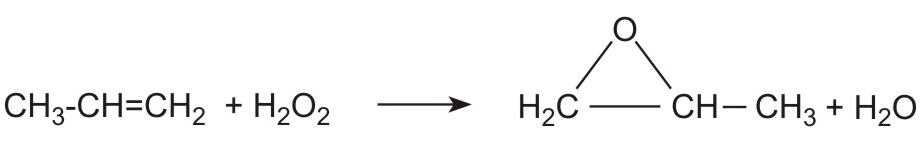
Alhough hydrogen peroxide is expensive to produce, the large scale of the plant, coupled with the lower costs associated with the eflfuent means that this new process is very attractive.
Manufacture of butanal (butrylaldehyde) and butanol
Butanal is produced by passing propene, carbon monoxide and hydrogen over a solid cobalt salt (a process known as the OXO process or hydroformylation):
(The isomer of butanal, 2-methylpropanal, (CH3)2CHCHO, is also formed).
Butanal is hydrogenated to butanol.
Date last amended: 26th January 2017