The development of chemicals to protect agricultural crops is an important activity within the chemical industry. Without them, many crops would suffer dramatic losses. Some of these chemicals, the insecticides, are also very important in combating human and animal diseases. The environmental and toxicological properties of these chemicals have improved considerably over the last six decades. Research aims to produce chemicals that are not just potent but are specific for the required purpose, whilst not affecting the environment in any other way. Because pests may develop resistance to crop protection chemicals there is a continual need for new products to be developed.
Three groups of chemicals dominate this part of the chemical industry (Figure 1). They are:
Herbicides: substances that kill or inhibit growth of unwanted plants (weeds)
Insecticides: substances that kill arthropod pests, i.e. insects and mites
Fungicides: substances that destroy or prevent the growth of pathogenic fungi
All three are pesticides.
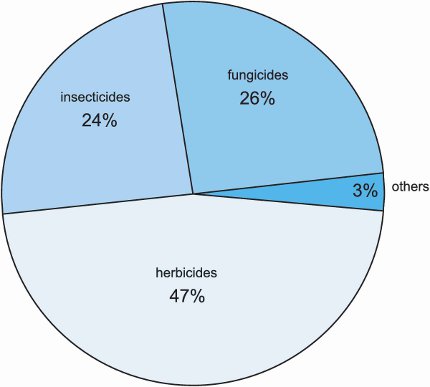
Figure 1 Global sales of crop protection chemicals (2008).
The effectiveness of a pesticide is the result of the proper 3D-assembly of specific groups in the chemical structure of its active ingredient. If several compounds of a given chemical class have related efficacies, they include a set of groups as a common minimum basic feature responsible for the best fit for the specific molecules in the biochemical target molecule (e.g. a protein) of the pest. This set of groups is called the toxophore. Where possible, the toxophores described in this unit are indicated by shading.
Development of new chemicals
It is estimated that it costs about œ150-200 million to discover a new product, test it thoroughly for its action and its safety for the environment, and develop manufacturing techniques for its synthesis. It takes an average of 10 to 15 years to do this so it is small wonder that, worldwide, only about 12 chemicals are introduced each year. However, these chemicals are key to the efficient production of food (Figure 2).
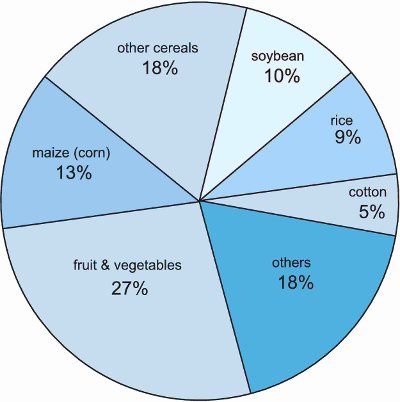
Figure 2 Sales value of crop protection chemicals worldwide by type of crop (2008).
Sometimes mimicking nature's own way of protecting plants can provide the answer to cost-efficient synthesis of effective crop protection chemicals. The pyrethroids, used as insecticides, are examples of this process. Another is a class of fungicides, the strobilurins, which mimic the natural fungicide strobilurin, a derivative of p-methoxypropenoic acid.
There is a continual search for pesticides with reduced risk. These are used in small amounts, are not susceptible to pests developing resistance, and have low toxicity for non-target organisms (humans, birds, fish and plants). Ideally non-target organisms do not have the same target which is affected by the pesticide or do not share the target's vulnerability that is exploited by the pesticide.
There are many hundreds of pesticides in use and being developed. This unit describes some specific examples currently being used from each of the three groups -herbicides, insecticides and fungicides.
Herbicides
Herbicides are used to control the growth of unwanted plants (weeds). Modern herbicides generally act by restricting growth. They inhibit the action of one or more of the many receptors that catalyze reactions which are essential to the growth of the plant. There is one group however, the auxins, that kill by overstimulating growth. With selective herbicides, either the target in the weed is affected more than that of the crop, the herbicide is degraded more quickly within the crop, or the uptake or translocation of the active ingredient differs from that of weeds. Non-selective herbicides kill crops as well as weeds.
Herbicides can act in several ways:
direct contact with plant tissues, for example, leaves; paraquat is a typical contact herbicide
- by translocation (systemic herbicides),whereby the compound has the ability to be absorbed by aerial plant parts and is transported to roots (basipetal translocation); glyphosate and growth hormones belong to this group
- by root uptake and transportation, to the upper parts of the plant (acropetal translocation)
- through a combination of both methods; triketones are an example of this group as they are transported downwards and upwards
(a) Bipyridyliums
These are non-selective herbicides that act by interfering with photosynthesis. Paraquat is one of the most used:
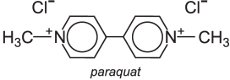
Although extremely poisonous if swallowed, it binds tightly to soil and is quickly deactivated. It is sold under trade names such as Weedol and Gramoxone.
(b) Auxins
Auxins are plant hormones. The most important member of the family is indole-3-ethanoic acid (indole-3-yl)acetic
acid, IAA).
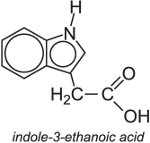
They act by promoting growth of the plant but, given high doses, this can kill the plant and thus act as an herbicide. An example of a synthetic auxin is 2,4-dichlorophenoxyethanoic acid, often known as 2,4-D.
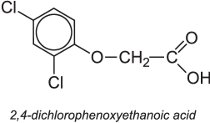
They are often used as one of the salts or esters. They are systemic herbicides and once absorbed act by translocation.
Two commercially sold auxins which mimic IAA are picloram and aminopyralid. They are both selective to broad leaved plants which absorb the herbicide through their leaves and translocate it to the roots. They can be used to kill weeds such as docks, thistles, dandelions and nettles amongst others.
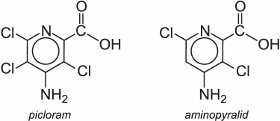
They are degraded by action of microorganisms in the soil and photochemically on the leaf of the plant.
(c) Glycines
Glyphosate ((N-phosphonomethyl)glycine) is a non-selective systemic compound useful in the control of broad-leaved weeds and grasses. It is by far the most widely used herbicide with annual sales well in excess of £3 billion.
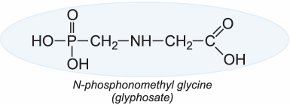
The herbicide is absorbed by the leaves and then translocates to the roots. By inhibiting the action of an enzyme it prevents the production of aromatic amino acids needed by the plants for protein synthesis. As this enzyme is absent in mammals, glyphosate has little toxicity. It is very effective and is rapidly degraded in the soil and by light. It is marketed under many names including Roundup and Tumbleweed.
Although glyphosate is a non-selective herbicide, selective seeds of corn (maize) and soya have been developed (via genetic engineering) whose plants can withstand the herbicide. Thus, fields can be sprayed with the herbicide and these transgenic crops will be the only ones that will not be affected. These seeds are used in many parts of the world but have not been allowed in most EU countries, except in very tightly controlled trials because of public concern about the effect of transgenic crops on the environment.
There have been reports that some weeds have become resistant to glyphosate. However some crops, for example soya, are being developed which are also resistant to auxins such as 2,4-D and so farmers will be able to use a mixture of glyphosate and an auxin.
Glufosinate is another non-selective herbicide.
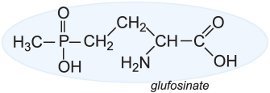
| Figure 3 Glufosinate inhibits the formation of glutamine synthetase, an enzyme that forms the amino acid glutamine from glutamate, a process vital for photosynthesis. The weeds around these transgenic soybean plants in the US have been controlled by spraying the whole field with a solution of glufosinate, marketed as Ignite. By kind permission of Bayer CropScience. |
 |
(d) Sulfonylureas
Characteristic features of these chemicals are their potency on a wide range of weeds and their safety to mammals and the environment. They act in plants to block the biosynthesis of essential amino acids (valine and isoleucine), and thus prevent cell division and cell growth.
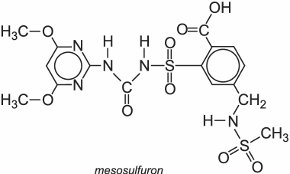
Mesosulfuron is another example of a herbicide which controls weeds in cereals and is marketed for example as Atlantis.
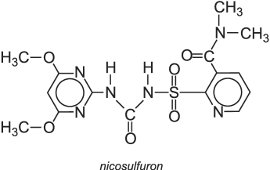
They are selective systemic herbicides which are absorbed by the foliage before translocating through the plant. They are particularly useful in controlling annual grass and dicot weeds in crops such as corn (maize) and are rapidly degraded in soils.
A well known example is nicosulfuron which is marketed, for example, as Accent which is used to control weeds in corn.
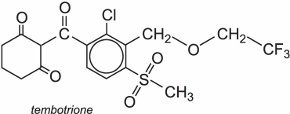
(e) Triketones
The triketones act by inhibiting an enzyme that would have led to the formation of compounds needed in the biosynthesis of carotenoids. These, in turn, play a key role in absorbing light energy for photosynthesis and protecting chlorophyll from photochemical damage. Triketones act through application on the foliage or via the roots. In either case there is translocation through the plant. They are particularly effective in controlling a wide range of weeds. Degradation in soils is fairly rapid.
Tembotrione is an example that allows the control of grasses and broad leaves in corn (Figure 4) and is sold under the trade name Laudis.
| Figure 4 The weeds in this field of corn (maize) have been controlled with tembotrione. It has been applied in combination with a compound that triggers degradation of tembotrione in the corn but not in the weeds. Such compounds are known as safeners. By kind permission of Bayer CropScience. |
 |
Mesotrione is another example of this class of herbicide and is sold under various trade names, including Callisto.
(f) Inhibitors of acetyl-CoA carboxylase
Some herbicides act by inhibiting the action of the enzyme, acetyl-CoA carboxylase, essential for the biosynthesis of carboxylic (fatty) acids, needed by the plant for the production of cell membranes. An example is pinoxaden which is particularly useful in dealing with grass in fields of barley and wheat.
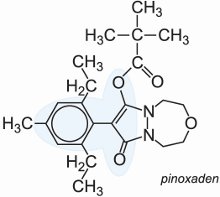
The inhibitors are selective herbicides which are absorbed by the leaves of the plant and act following translocation. They are rapidly degraded in the soil.
Among the trade names for pinoxaden is Axial.
Insecticides
The world market for insecticides is dominated by compounds interfering with the nervous system of pest invertebrates, since this target organ usually provides rapid control. Insecticides acting on target sites such as acetylcholinesterase (organophosphates and methyl carbamates), voltage-gated sodium channels (pyrethroids), nicotinic acetylcholine receptors (neonicotinoids) and ligand-gated chloride channels (macrocyclic lactones and phenylpyrazoles) account for more than 75% of total insecticides sales (Figure 5).
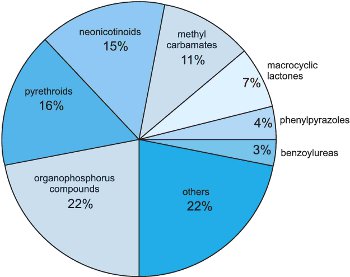
Figure 5 World market share of insecticides, by class (2008).
Other mechanisms such as interference with insect development are usually more selective (either by distinguishing between species or by their life cycles), but such compounds are generally much slower in their action.
 |
| Figure 6 Uncontrolled aphids (whitefly) attacking newly planted beech trees. By kind permission of Dr Allan Clements. |
(a) Organophosphorus compounds
First introduced in 1944, organophosphorus compounds (OPs) are economically still the most successful and diverse chemical class of insecticide. More than 100 different active ingredients belonging to this class are known. One of the most successful OPs is considered to be chlorpyrifos. This non-systemic insecticide, used on both the foliage and the soil, affects the stomach and respiratory action of the pest.
All OPs act by binding irreversibly to the enzyme, acetylcholinesterase (AChE). This prevents the hydrolysis of the neurotransmitter acetylcholine in the central nervous system (CNS) and leads to prolonged periods of nerve excitation. This then results in paralysis and subsequently death of the insects and their predators. OPs are used to control almost all pests including Lepidoptera (e.g. moths), Coleoptera (e.g. beetles), Diptera (e.g. flies, such as whiteflies, and mosquitoes), Hemiptera (e.g. aphids and leafhoppers). Additionally they control nematodes (parasitic worms) and mites. Four examples are:
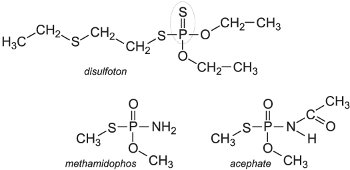
The major disadvantage of most OPs is their toxicity to vertebrates and this has resulted in the search for other compounds to replace them.
Organophosphorus compounds are rapidly broken down by enzymatic action in the soil.
(b) Methyl carbamates
Another important class of AChE inhibitors, introduced in the late 1950s, is the methyl carbamates which are usually less toxic to non-target organisms than the organophosphorus compounds.
Their structural features are found in a natural product isolated as early as 1864, physostigmin (eserin), the structure of which was finally elucidated in 1925.
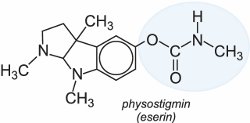
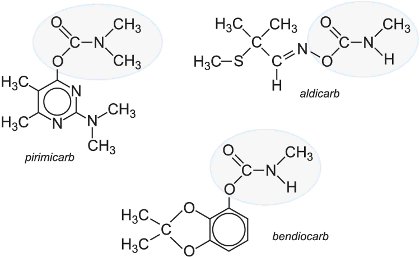
They have short to moderate effective life, but are more selective in their effect. Pirimicarb is one of the most important methyl carbamates for aphid control. It was launched as a foliar spray for vegetables, arable crops and fruits in the early 1970s and is harmless to pollinating insects and aphid predators. Other widely applied carbamates are aldicarb which is used primarily as a soil insecticide and bendiocarb which is very important for the control of malaria-transmitting adult mosquitoes.
(c) Macrocyclic lactones (avermectins and milbemycins)
All natural and semi-synthetic 16-membered macrocyclic lactones disturb the systems which control the flow of chloride ions. This shuts down the electrical impulses in the nerve cells of their target organisms.
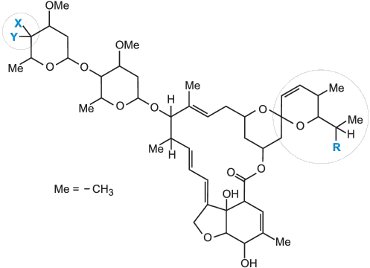
| X | Y | R | |
|---|---|---|---|
| avermectin B1 | OH | H |
>80% C2H5 <20% CH3 |
| emamectin benzoate | H | NH-CH3 |
>90% C2H5 <10% CH3 |
The naturally occurring avermectins and emamectin benzoate are based on the above structure. The avermectins are produced by fermentation from the soil microorganism Actinomycetes (from the genus Streptomyces) and emamectin benzoate is prepared from abamectin in a series of chemical reactions. Another naturally occurring example is milbemycin.
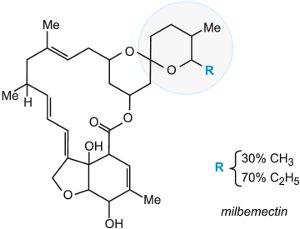
The whole family of macrocyclic lactones displays unprecedented potency against mites and insects as well as nematodes (e.g. parasitic worms). Avermectin is used in various crops such as citrus, pome fruits (for example, apples and pears), vegetables and cotton. Milbemectin is mainly used to combat the many different mites in tea and pome fruits (e.g.apples), and also against pine wood nematode which devastates pine trees in Japan and parts of the US.
The products are non-systemic and are removed rapidly from the environment after application. Photolysis on plant surfaces is fast, and they bind tightly to soil, where they are rapidly degraded by soil microorganisms. Thus, no leaching or bioaccumulation occurs. Because of the rapid uptake into sprayed foliage combined with fast degradation of surface residues, this family of insecticides is safe to use.
(d) Phenylpyrazoles
A well-known member of this class of insecticides is fipronil, which can be applied to the foliage, soil and seeds. However, it has a limited ability to translocate through the plant. Fipronil acts by disturbing the chloride ion concentrations in cells in pest species such as the Lepidoptera (moths), Coleoptera (beetles) and Diptera (flies, mosquitoes). For example, it is widely used as a foliar spray for the control of leaf- and planthoppers in rice in south-east Asia.
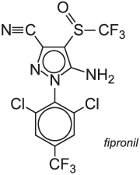
Other applications of the phenylpyrazoles include the control of urban pests such as ants and cockroaches indoors and on lawns. They are also very effective in controlling termites. In animal health care, they are used on cats and dogs to combat ticks and fleas.
(e) Nereistoxin analogues and neonicotinoids
Much work has been devoted to finding chemicals which will prevent the nicotinic acetylcholine receptor (nAChR) from functioning correctly. The receptor is important in allowing the passage of sodium and potassium ions in the nerve cells and thus affects the central nervous system. First examples of these insecticides are cartap, thiosultap, bensultap and thiocyclam.
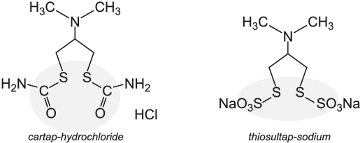
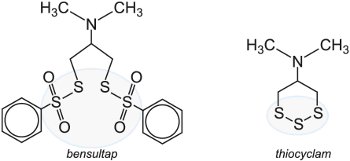
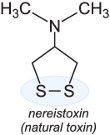
They break down either by action of water or light to produce the toxin, nereistoxin, a substance that was first isolated from the naturally occurring marine nereid worm Lumbrineris heteropoda Marenz, which acts by effectively paralyzing the pest.
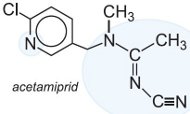
Another group of insecticides which affect the nicotinic acetylcholine receptor is the neonicotinoids which are now the fastest growing and fourth major class of insecticides in crop protection. They are active against a broad range of insect pests, and exhibit activity through both oral (ingestion) and contact routes of application. They have a high level of efficacy, and a favourable environmental and toxicological profile. This has led to their rapid adoption in numerous agricultural areas for quick control of a broad range of chewing and sucking pests with minimum impact on beneficial insects.
Like the naturally occurring alkaloid nicotine (used for a long time in the form of aqueous tobacco extract),
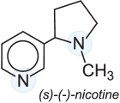
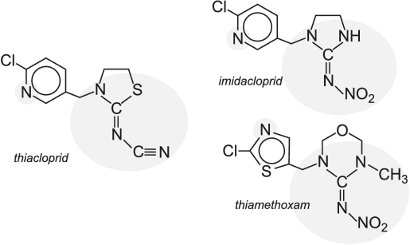
neonicotinoids act selectively on and overstimulate the insect's central nervous system. The following are examples of this class of pesticide:
 |
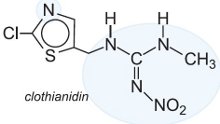 |
They have been shown to have long-lasting effect, both when treating the leaves and the seeds. Further, they have a relatively low risk for non-target organisms, although recently there have been concerns that they may be contributing to the significant decline in honey bee populations. The environment agencies in several countries are currently assessing the evidence for this.
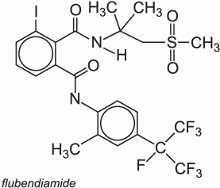
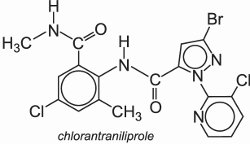
(f) Diamides
Diamides represent a new class of insecticidal chemistry, which include the phthalic acid diamides and the anthranilic diamides (chlorantraniliprole). They act on the cell's ability to control the flow of calcium ions (ryanodine receptors).
Flubendiamide is an example of a phthalic acid diamide:
Chlorantraniprole is an example of an anthranilic diamide:
 |
| Figure 7 Rynaxypyr® is used to protect cabbages from the ravages of caterpillars. By kind permission of DuPont. |
It is marketed under several names, for example Rynaxypyr® (Figure 7).
The ryanodine receptor as an insecticide target site has been known for decades and is named after the natural plant-derived (alkaloid) insecticide, ryanodine. Although ryanodine's insecticidal properties are limited under field conditions, diamide insecticdes have been developed which are specific to insect ryanodine receptors and are particularly active against Lepidoptera (moths). Diamides protect fruit and vegetables from beetles, weevils, leaf miners and caterpillars.
They do not affect mammalian ryanodine receptors, most probably explaining their excellent toxicological profile, being specific to the pests and not affecting natural predators or pollinators and being non-toxic to mammals, fishes and birds.
(g) Pyrethroids
Among the most important chemical classes of insecticides is the pyrethroids, which affect the flow of sodium ions in the nervous system. In the middle of the 19th century an insecticidal powder derived from dried flower heads of the genus Pyrethrum (Chrysanthemum) was introduced from Africa to central Europe. The insecticidal components were identified as pyrethrins. As they have several asymmetric centres these compounds have many stereochemical forms, and only a few of them are active as an insecticide. Natural pyrethrins I and II are unstable, sensitive to photo-degradation and relatively expensive. However, these natural pyrethrins were used as templates to generate analogues, the so-called synthetic pyrethroids. They act on contact with the plant.
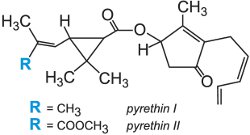
They can be separated into two classes according to the poisoning action they induce. Type I pyrethroids (e.g. permethrin) cause hyperactivity and incoordination, whereas Type II pyrethroids (e.g. deltamethrin) containing an alpha-cyano substituent induce nerve depolarization (polarization being needed to transmit the electrical signals in the nervous system) and subsequently paralysis of the insect. Pyrethroids are highly active against Lepidoptera pest species, but their speed of action, which leads to rapid knock-down, means they are used successfully in many crops against numerous pests including aphids.
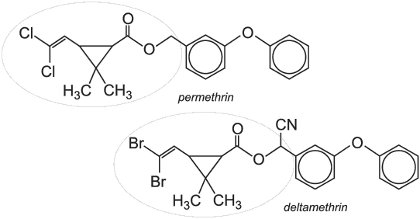
Pyrethroids are very useful insecticides as, although toxic to insects, they are harmless to mammals. They are non-systemic and are rapidly broken down in the soil. They are also used as indoor residual spray or on insecticide-treated nets for the control of malaria mosquitoes.
(h) Benzoylureas
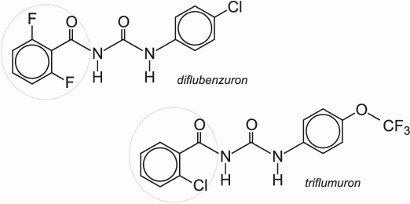
Benzoylureas have been developed and used as commercial insect growth regulators (IGRs) acting by inhibiting the biosynthesis of chitin (a long chain polymer of N-acetylglucosamine which is in the cell walls of the insect). Diflubenzuron was one of the first of this class to be used commercially when it was introduced in 1977. It is used for the control of chewing insects and coleopteran pests (beetles and weevils) in fruit, cotton, soybeans and vegetable crops.
Because of their non-toxicity to vertebrates, the benzoylureas such as lufenuron and triflumuron are also used in veterinary medicine and in the home against animal and human health pests such as fleas, ticks and cockroaches.
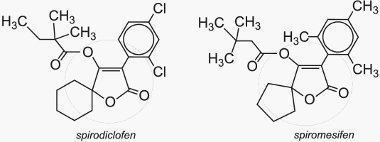
(i) Ketoenols
Ketoenols are a new chemical class of insecticides affecting development by inhibiting acetyl CoA carboxylase and subsequent lipid biosynthesis. Spirodiclofen is a new non-systemic foliar acaricide (kills mites and ticks). It has particularly excellent long lasting efficacy and is effective in early to late season applications for spider mite control. Spirodiclofen is being developed for worldwide use in pome fruit (e.g apples, pears), stone fruit, citrus fruit, grapes, almonds and nuts, being very effective against mites. Spiromesifen is a new foliar contact acaricide and has been used worldwide on vegetables, fruits, cotton, corn, beans, tea and some ornamentals.
Fungicides
The term fungicide normally applies to synthetic chemical compounds that kill fungi or inhibit their growth. However, certain biological organisms can also be used to control fungal infections, which include mildews, rusts and leaf spots.
 |
 |
 |
| Figure 8 Some problem fungi on important crops. From left to right: potatop blight in its early stages; apple scab; grey mould on grapes. By kind permission of Bayer CropScience. |
Some fungicides offer protection against the development of fungi (termed protective), and others cure the plant by eliminating the fungus (termed curative).
Nearly all modern fungicides (except for inorganic salts, see below) degrade in aerobic soils and therefore they are environmentally friendly, the rate of degradation depending on the temperature and humidity.
Since fungi rapidly develop resistance to these chemicals, it is necessary to be able to draw on fungicides from different chemical classes and with different sites of action. The compounds discussed here are representatives of each important chemical class but the list is not exhaustive.
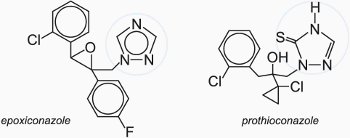
(a) Triazoles
The 1,2,4-triazoles are one of the most important classes of fungicide.
They offer protection against a wide range of fungi that occur in cereals, rice, beet, trees, vegetables and flowers. These include amongst others powdery mildews, leaf spot diseases and rusts. The triazoles inhibit important biochemical pathways that produce sterols which are essential components of cell membranes.
The compounds are applied to the leaf surface of the plant and are distributed all over the plant (systemic activity). They act by both preventing the action of the fungi by inhibiting the germination of the spores and also curing the problems caused by the fungi. They are thus both protective and curative.
Examples of commercially available triazoles include:
(b) Strobilurins
The development of a chemical class called strobilurins was inspired by the study of a group of naturally occurring fungicides, for example, strobilurin A.
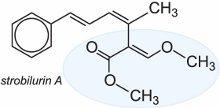
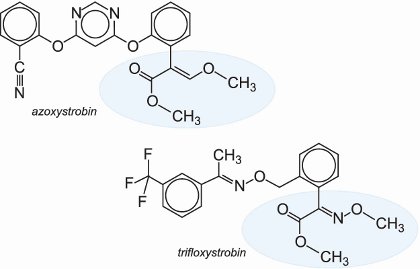
Strobilurins interfere with the production of adenosine-5'-triphosphate (ATP), the nucleotide that transports energy within cells for metabolism, thus preventing germination and growth. The strobilurins can combat most major fungal diseases found on grasses (turf), vines, fruit and particularly cereals.
Like the triazoles, they are systemic, and they offer protection and also cure the problems caused by the fungi.
Examples of commercially available strobilurins are given below:
(c) Carboxamides
Carboxamides are so named as they contain the carboxylic acid amide (-CO-NH2) or related group. As with the strobilurins, they interfere with the production of ATP.
An example of a carboxamide fungicide is boscalid used in particular to control powdery mildew on fruit and vegetables.
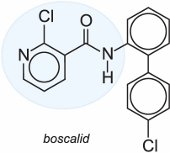
It is expected that several other compounds in this class will be commercially available in the near future examples of which are bixafen and isopyrazam.
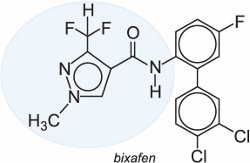 |
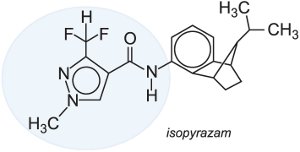 |
(d) Fungicides with a 'multi site action'
Besides fungicides which act mainly on one target enzyme, several fungicides with a 'multi site action' are commercially available. They act unspecifically on a number of enzymes and are therefore not very sensitive to resistance problems. They are all protectant fungicides, acting on contact with the fungal spores on the surface of the leaves.
Some examples are inorganic compounds such as elemental sulfur and some copper salts of which the 'Bordeaux mixture' (a mixture of copper(II) sulfate and calcium hydroxide) is the most famous. Both are allowed to be used in the production of organic food. Examples of organic compounds with a multi site action are salts of dithiocarbamates such as chlorothalonil and propineb. They are very widely used on a wide range of plant pathogens in fruits and vegetables such as grapes, apples, tomatoes and potatoes.
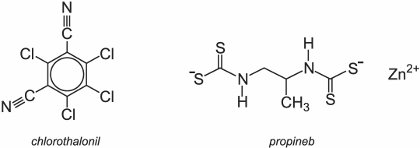
(e) Specific fungicides with activity against downy mildews
Since downy mildews are a special class of plant pathogens causing severe damage mainly in grapes and potatoes a number of specific fungicides active only against this kind of plant diseases are available. Most of them act systemically. Since they belong to different chemical classes they exhibit several modes of action.
Examples are metalaxyl and iprovalicarb:
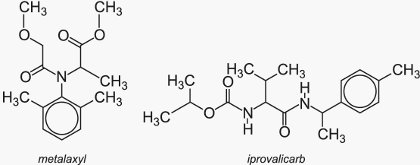
Date last amended: 18th March 2013
