.jpg) Ethane-1,2-diol, (ethylene glycol, monoethylene glycol, MEG) which is manufactured from ethene via epoxyethane, is used to make polyester fibres, resins and films although it is probably better known for its use as a coolant in cars. It is miscible with water and it lowers the freezing point of water so it is used as an antifreeze.
Ethane-1,2-diol, (ethylene glycol, monoethylene glycol, MEG) which is manufactured from ethene via epoxyethane, is used to make polyester fibres, resins and films although it is probably better known for its use as a coolant in cars. It is miscible with water and it lowers the freezing point of water so it is used as an antifreeze.
Uses of ethane-1,2-diol (ethylene glycol)
Figure 1 Uses of ethane-1,2-diol.
Data for 2013 from a variety of sources including IHS Markit, 2016.
By far the most important uses of the diol is in the manufacture of polyesters, particularly PET (polyehylene terephthalate), used widely for clothes and for packaging. Indeed 45% of the polyester is used for bottles1.
1. Global Processing, 2016.
Another important use is as a coolant in engines. In modern engines, higher running temperatures mean better fuel efficiency and reduced emissions. Water is by far the best coolant, having a low viscosity, high specific heat capacity and high thermal conductivity, but is limited in its use because of its relatively high freezing point, low boiling point and it corrodes metals. Ethane-1,2-diol (antifreeze) mixed with water up to 60% volume gives a solution with a freezing point down to 223 K. The boiling point of the mixture also increases.
The corrosion problem is addressed with corrosion inhibitors. If only cast iron had to be protected, a simple pH adjustment with sodium hydroxide and sodium borate as a buffer could suffice, but engines are complex systems of many metals and alloys so inhibitors are packages of a number of compounds each of which has a particular function. A traditional European package might contain sodium hydroxide and sodium borate for pH control to 7-9 (corrosion is much slower under alkaline conditions), sodium nitrate, sodium silicate, tolyltriazole, an organic acid (such as benzoic, sebacic or citric), and sometimes a compound which prevents foaming.
The globalisation of the car industry is leading manufacturers to search for a 'world coolant', acceptable everywhere.
Annual production of ethane-1,2-diol (ethylene glycol)
| World | 18 million tonnes |
| Europe | 5 million tonnes |
| North America | 3 million tonnes |
| US | 1.1 million tonnes1 |
1. 2015, Business of Chemistry, American Chemistry Council, 2016
Manufacture of ethane-1,2-diol (ethylene glycol)
Epoxyethane, produced from ethene, reacts with water to form ethane-1,2-diol:
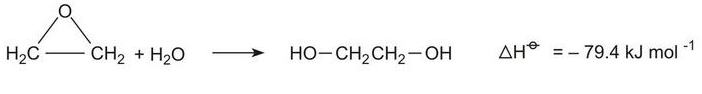
The hydration takes place under a variety of conditions, neutral, acid-catalysed and base-catalysed. Usually the reaction in industry is carried out in neutral conditions or is catalysed by acid (0.5% sulfuric acid) at 320-340 K, under pressure.
Formation of higher homologues is inevitable, because epoxyethane reacts faster with diols than with water. This can be minimised by using a large excess of water. A 20-fold molar excess is not unusual. In practice, up to 90% of the epoxyethane can be converted to ethane-1,2-diol. Recently, over 93% yields have been obtained.
After leaving the reactor, the product is distilled at lower pressures to remove water which is returned to the reactor. Ethane-1,2-diol is then purified by vacuum distillation. Heat from the reactor is used to heat the distillation columns.
This production method is simple but has some disadvantages, such as requiring corrosion resistant materials for the plant, the removal of the acid from the product and the distillation of large volumes of water.
A process has been developed recently for converting epoxyethane to ethane-1,2-diol with a yield of well over 99%. It is based on technology from Mitsubishi Chemicals and further developed by Shell Chemicals.
The conversion from epoxyethane to the diol takes place in two stages. First the epoxide reacts with carbon dioxide to form 1,3-dioxolan-2-one (ethene carbonate):
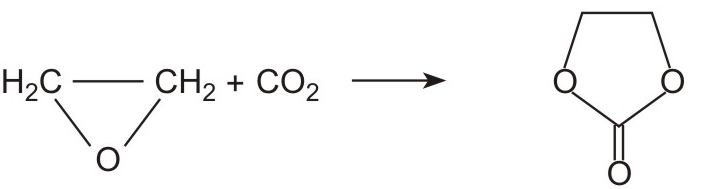
An organophosphorus compound is used as the catalyst.
Ethene carbonate is then reacted with water to form ethane-1,2-diol and regenerates carbon dioxide.
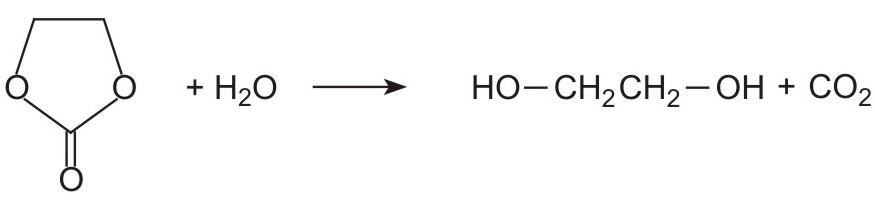
At first sight, it might be expected that the one stage process from epoxyethane to the diol would be more cost-effective than a two-stage process involving carbon dioxide. However, the selectivity of the one stage direct hydration process, in terms of producing ethane-1,2-diol rather than other by-products (higher diols), is at best 93%. Whereas with the two stage process, the selectivity increases to over 99.5% which is sufficiently high for there to be no need for a further stage of purification of the diol from the by-products .Further, once the two stage process is running, the carbon dioxide is recycled very efficiently.
 |
| Figure 2 As with all chemical processes used in industry, the scale up from laboratory conditions on the gram scale to a full scale industrial plant, in which thousands of tonnes are produced annually, is very painstaking work for chemists and chemical engineers. This photograph shows an intermediate stage in which a small plant, the pilot plant, has been made to mimic large scale conditions for the OMEGA Process to produce ethane-1,2- diol. With kind permission of Shell International Ltd. |
Shell now sells a combined EO/MEG technology as an integrated Omega (only mono-ethylene glycol advanced) process package taking ethane and converting it to ethane-1,2-diol.
The Omega process not only has lower capital costs because there is no need for the purification equipment to remove by-products, but the operating costs are also reduced as it uses much less water, consequently lowering power and water treatment costs.
The first plant using the Omega Process was commissioned in South Korea in 2008.
 |
| Figure 3 A new integrated refinery and petrochemicals facility is under construction in Singapore, known as the Shell Eastern Petrochemicals Complex. It will include a very large steam cracker to produce ethene together with additions to the existing refinery on Bukom Island. A new plant to produce ethane-1,2-diol using the Shell OMEGA process, is being built on nearby Jurong Island. Bukom and Jurong Ilands are close together, about 5 kilometres south of the tip of Singapore. With kind permission of Shell International Ltd. |
Date last amended: 8th November 2016

