Surfactants are one of many different compounds that make up a detergent. They are added to remove dirt from skin, clothes and household articles particularly in kitchens and bathrooms. They are also used extensively in industry. The term surfactant comes from the words surface active agent.

Figure 1 Surfactants aid the effective washing of dirty rugby kit using low
temperature wash cycles, resulting in environmental benefits.
By kind permission of Stephen Garnett/Wharfedale RUFC.
Surfactants function by breaking down the interface between water and oils and/or dirt. They also hold these oils and dirt in suspension, and so allow their removal. They are able to act in this way because they contain both a hydrophilic (water loving) group, such as an acid anion, (-CO2- or SO3-) and a hydrophobic (water hating) group, such as an alkyl chain. Molecules of water tend to congregate near the former and molecules of the water-insoluble material congregate near the latter (Figure 2).
Soaps were the earliest surfactants and are obtained from fats which are known as glycerides because they are esters formed by the trihydric alcohol, propane-1,2,3-triol (glycerol), with long chain carboxylic acids (fatty acids). The glycerides are hydrolyzed by heating with sodium hydroxide solution to form soaps, the sodium salts of the acids, and propane-1,2,3-triol. The process is known as saponification.
.jpg)
.jpg)
Figure 2 Action of a surfactant.
Manufacture
The glycerides used to make surfactants contain saturated and unsaturated carboxylic acids which have an even number of carbon atoms, generally within the range 12-20, for example, octadecanoic acid (stearic acid), CH3(CH2)16CO2H.
Synthetic surfactants have one very important advantage over soaps. Because soaps form insoluble calcium and magnesium salts with the calcium and magnesium ions in hard water and in the clays which are present in dirt, much of the soap is wasted forming an insoluble scum. However, this is avoided when using a synthetic surfactant. For example, in the anionic surfactants, the carboxylate group in soap is replaced by a sulfonate or sulfate group as the hydrophilic component. The corresponding calcium and magnesium salts are more soluble in water than the calcium and magnesium salts of carboxylic acids.
Surfactants are classified based upon the nature of the hydrophilic "head-groups" as:
- anionics cationics
- nonionics
- amphoterics
Anionic surfactants
In these surfactants the hydrophilic group is negatively charged. They are the most widely used type of surfactants for laundering, dishwashing liquids and shampoos. They are particularly good at keeping the dirt, once dislodged, away from fabrics.
Four anionic surfactants are used:
a) alkylbenzene sulfonates
b) alkyl sulfates
c) alkyl ether sulfates
d) soaps
(a) Alkylbenzene sulfonates
The most common of the synthetic anionic surfactants are based on the straight chain alkylbenzene sulfonates. Benzene, in slight excess, is mixed with an alkene or chloroalkane in the presence of an acid catalyst, usually a solid zeolite (ion exchange), aluminium chloride (AlCl3) or hydrofluoric acid (HF), to produce an alkylbenzene (sometimes called detergent alkylate).
For example:
.jpg)
The alkylbenzene varies in average molecular mass, depending upon the starting materials and catalyst used and is often a mixture in which the length of the alkyl side chain varies between 10 and 14 carbon atoms. Historically these included branches in the side chains with the result that they biodegrade very slowly and lead to foaming in rivers and sewage plants. By law, in most countries today, the surfactant must have side chains which are not branched so they degrade more rapidly.
The alkylate is sulfonated using an air/sulfur trioxide mixture, and the resulting sulfonic acid is then neutralised with an aqueous solution of sodium hydroxide (often in situ), for example:
.jpg)
Straight chain alkenes for the above process can be produced from ethene using a Ziegler catalyst (triethyl aluminium). Triethyl aluminium reacts with ethene at ca 400 K and 100 atm to form aluminium alkyls, for example:
.jpg)
When heated in excess ethene, straight chain alkenes, with the double bond at the end of the chain (an a-alkene), are produced:
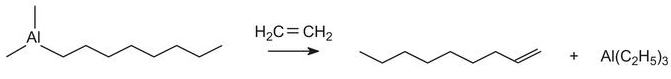
The mixture is then separated into fractions by distillation, the fraction of alkenes containing 10 to 14 carbon atoms being used to make the surfactants.
These are used together with other surfactants in powder and liquid laundry detergents such as Ariel, Daz, Persil and Surf.
(b) Alkyl sulfates
.jpg)
Many detergent products, particularly liquids, contain other synthetic anionic surfactants such as alkyl sulfates, esters of linear alcohols (C10-C18) and sulfuric acid. The alkyl sulfates are also used in personal care products such as toothpaste and are manufactured by treating the alcohol with sulfur trioxide. The product is then neutralised with aqueous sodium hydroxide solution to form a sodium alkyl sulfate:
.jpg)
The alcohols are either produced from carboxylic acids obtained from oils, obtained naturally, for example from palm kernel oil or coconut oil, or alternatively from long-chain alkenes, manufactured from ethene.
There are two processes for making the alcohols from ethene. As described above, aluminium triethyl reacts with ethene to produce compounds such as:
.jpg)
where a,b,c are even numbers from 2 to 12. Instead of heating with excess ethene to produce a-alkenes, the aluminium alkyl is treated with oxygen and then water to produce long chain alcohols:
.jpg)
Alternatively, a different process for making the alcohols from ethene is used, known as SHOP (Shell Higher Olefins Process). In the first stage, ethene is passed, under pressure of ca 100 atm, into a solvent (usually a diol, such as butane-1,4-diol) containing a nickel salt at 400 K. It yields a mixture of a-alkenes which are separated by fractional distillation. About 30% are in the range C10-C14.
These are reacted with carbon monoxide and hydrogen (hydroformylation) to yield straight-chain aldehydes, which on reduction form alcohols. For example:
.jpg)
It is possible to convert the other a-alkene fractions (C4-C10 and C14-C40) into the more desirable C10-C14 fraction.
(c) Alkyl ether sulfates
More widely used than simple alkyl sulfates are various types of sodium alkyl ether sulfates (SLES).
In the manfacture of SLES the primary alkyl alcohol (from a synthetic or natural source and typically a blend based around dodecanol) is first ethoxylated with 1 to 3 molar equivalents of epoxyethane (as described below for the manufacture of nonionic surfactants). The product is then sulfated using sulfur trioxide and neutralized with alkali to form the alkyl ether sulfate:
.jpg)
These materials are preferred by product formulators for many applications (dishwashing liquids, shower gels, shampoo, etc) because they are milder to the skin than alkyl sulfates. They also generate less foam which is an advantage in the formulation of laundry machine products.
(d) Soaps
Soaps are anionic detergents:
.jpg)
Cationic surfactants
With these surfactants, the hydrophilic head is positively charged.
Although they are produced in much smaller quantities than the anionics, there are several types, each used for a specific purpose.
(a) Mono alkyl quaternary systems
The simplest quaternary system is the ammonium ion:
.jpg)
An alkyl quaternary nitrogen system has alkyl groups attached to the nitrogen atom. An example is:
.jpg)
They are used as fabric softeners with anionic surfactants, helping them to break down the interface between the dirt/stain and the water.
(b) Esterquats
The directly quaternised fatty acid surfactants described above have been replaced for laundry applications by more complicated structures in which there is an ester linkage between the alkyl chains and the quaternary head-group as these are more biodegradable and less toxic. They are known as esterquats.
An example is:
.jpg)
Esterquats give detergents their fabric softening qualities.
Nonionic surfactants
These surfactants do not bear an electrical charge and are often used together with anionic surfactants. An advantage is that they do not interact with calcium and magnesium ions in hard water.
They account for nearly 50% of surfactant production (excluding soap). The major group of nonionics are the ethoxylates made by condensing long chain alcohols with epoxyethane (ethylene oxide) to form ethers, for example:
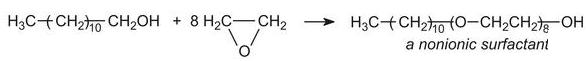
The long-chain alcohol can come from either a synthetic or natural source.
Although they do not contain an ionic group as their hydrophilic component, hydrophilic properties are conferred on them by the presence of a number of oxygen atoms in one part of the molecule which are capable of forming hydrogen bonds with molecules of water.
.jpg)
As the temperature of the surfactant solution is increased the hydrogen bonds gradually break causing the surfactant to come out of solution. This is commonly referred to as the cloud point and is characteristic for each nonionic surfactant. Nonionics are more surface active and better emulsifiers than anionics at similar concentrations. They are less soluble than anionics in hot water and produce less foam. They are also more efficient in removing oily and organic dirt than anionics. Depending on the type of fibre, they can be active in cold solution and so are useful in countries which lack hot water supplies and in developed countries where there is a desire to lower the wash temperatures either to save energy or because of the type of fabric being washed. Nonionics are used in fabric washing detergents (both powders and liquids), in hard surface cleaners and in many industrial processes such as emulsion polymerization and agrochemical formulations.
Amphoteric surfactants
Amphoteric (or zwitterionic) surfactants are so called because the head-group carries both a negative and positive charge. A range of methods is used to produce such materials, almost all of which contain a quaternary ammonium ion (a cation). The negatively charged group can be carboxylate, -CO2-, sulfate, -OSO3- or sulfonate, -SO3-. One such well-used class is the alkyl betaines which have a carboxyl group. A long-chain carboxylic acid reacts with a diamine to form a tertiary amine. On further reaction with sodium chloroethanoate, a quaternary salt is formed:
.jpg)
Betaines are neutral compounds with a cationic and an anionic group which are not adjacent to one another.
.jpg)
Amphoteric surfactants are very mild and are used in shampoos and other cosmetics. They are said to be pH balanced.
Applications
Laundry detergents
A detergent is made up of many ingredients, some of which are surfactants. An example of the mixture of compounds in a detergent is shown in Table 1.
In this formulation there are seven surfactants, two anionic, three non-ionic and two soaps.
However, there are other ingredients, each with specific functions:
Bulking agents, such as sodium sulfate and water.
Some detergents need anti-caking agents, for example aluminium silicate, which keep the powder dry and free-flowing.
Builders, usually sodium aluminosilicates, a type of zeolite, remove calcium and magnesium ions and prevent the loss of surfactant through scum formation.
Stains can be bleached with oxidizing agents such as sodium perborate (NaBO3.4H2O) and sodium percarbonate (2Na2CO3.3H2O2) which react with hot water to form hydrogen peroxide which in turn reacts with the stain:
.jpg)
However bleach activators are needed for low temperature washes. Sodium perborate and sodium percarbonate do not liberate hydrogen peroxide in cool water. A compound is added to react with them to liberate a peroxycarboxylic acid, RCO3H, which oxidizes stains readily. The most commonly used activator is:
.jpg)
It is known by its trivial name, TAED, and reacts with the oxidizing agent to form peroxyethanoic acid:
.jpg)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Table 1 Ingredients of a detergent for washing clothes. |
Other ingredients which can be added to a detergent include:
Buffering agents - to keep the pH at the appropriate value
Structurants - to give shape to the fabric being washed
Sequestrants - to react with free metal ions which might otherwise cause problems with appearance or scum formation
Optical brighteners - to make the fabrics look brighter and whiter
Antifoaming agents
Enzymes - to remove specific stains: proteases (to remove proteins), amylases (to remove starches), lipases (to remove fats)
Fragrance
Anti-redeposition agents - to prevent dirt being redeposited on fabrics
Skin conditioning agent - to help to keep the skin in good condition
Softness extender - to help keep the clothes 'soft'
Emulsifier - to help keep immiscible liquids as an emulsion
Colorant
Domestic automatic machine laundry liquids are formulated using blends of anionic, nonionic and soap surfactants and various other functional substances. Bleach systems are not compatible with the higher water temperature and cannot be used above ca 315 K.
For hand washing (used for delicate fabrics such as wool or silk), foam-stabilisers are included, to maintain foam. The customer equates the quantity of foam produced with the detergent cleansing action. For the quantity of foam produced the order is:
anionics > soap > nonionics > cationics
Machine dishwashing powders and tablets
The products used in dishwashers are usually powders and contain builders (90-95%), a nonionic surfactant (1-5%), bleach agents with an activator and enzymes. They are formulated with sodium carbonate and sodium silicate to create a very alkaline environment that helps to denature (break down) the fats and proteins left on the used dishes and utensils.
Washing up liquids
These formulations contain between 13-40% of surfactants which are predominantly alkyl ether sulfates but also include nonionics and amphoterics (betaines).
Shampoos and shower gels
These tend to be based on alkyl ether sulfates and usually contain small amounts of other surfactants (most typically amphoterics) which help protect the skin from irritation and also condition the hair.
Hair conditioners and fabric softeners
These products are formulated using cationic surfactants (sometimes combined with small amounts of non-ionic surfactants). These are not cleansing products and the cationic surfactant is deposited onto the slightly negatively charged hair or cotton fibre surface, thus giving a lubrication benefit.
Environmental considerations
In Western Europe all surfactant components of domestic detergents must be biodegradable. This requirement resulted from the fact that the original alkylbenzene sulfonate anionics were based on branched alkenes and these proved resistant to degradation by bacteria at sewage treatment works causing many rivers to suffer from foam. There was also a fear that surfactants could be "recycled" into drinking water. Similar concerns were expressed about nonylphenol ethoxylates and so in the 1980s the industry moved to linear alkylbenzene sulfonates and alcohol ethoxylates as the major ingredients of their formulations. Effective sewage treatment ensures that detergent components which are part of household effluent water are not discharged untreated into rivers and water courses.
The development of compact powders and liquids and refillable packages is designed to reduce packaging waste.
Redesign of washing machines and laundry detergent products (including the addition of bleach activators and enzymes to ensure good stain removal at low temperatures) has resulted in energy savings by reducing water heating and using shorter wash cycles.
Date last amended: 18th March 2013
