.jpg) Methyl t-butyl ether (2-methoxy-2-methylpropane), often referred to as simply MTBE, has been widely used over the last 30 years to improve the burning characteristics of petrol.
Methyl t-butyl ether (2-methoxy-2-methylpropane), often referred to as simply MTBE, has been widely used over the last 30 years to improve the burning characteristics of petrol.
When oil is distilled, the fraction that can be used as petrol (the naphtha fraction) is made up mainly of C4 to C10 straight chain alkanes, which have a suitable volatility. However, these hydrocarbons burn in a way that leads to explosion in the engine cylinder at the wrong time (knock). If petrol vapour and air ignites in the cylinder of the car engine before the cylinder reaches the optimum position, a process known as pre-ignition and also as engine knock occurs. The term knock is used as pre-ignition can be heard. Severe knock can cause serious engine damage.
There are three ways of improving the performance of petrol (indicated by the octane rating of the petrol). One is to add a volatile lead compound, usually lead tetraethyl, Pb(C2H5)4 (TEL). However, there is great concern that when lead compounds are released into the atmosphere from the exhaust, they damage our health. They also poison the metal catalysts in catalytic converters. The use of lead compounds in petrol is now prohibited in most countries. The United Nations Environment Programme (Unep) has conducted a long campaign to eliminate leaded fuel from developing countries. According to the UN body, TEL-boosted petrol is now only used in six countries which include Afghanistan, Iraq, Yemen and North Korea.
Another way to reduce knock is to increase the proportion of branched chain alkanes, cycloalkanes (alicyclic) and aromatics in petrol. The branched chain alkanes, cycloalkanes and aromatics are produced from straight-chain alkanes in a variety of ways, for example, by catalytic cracking of gas oil and by reforming of naphtha. However, because of its toxicity, the use of benzene, one of the most effective hydrocarbons to help in raising the octane rating, is restricted.
A further way to improve the octane rating is to add an oxygenated compound. The favoured compounds are alcohols (methanol and ehanol) and one of three ethers MTBE (methyl t-butyl ether), ETBE (ethyl t-butyl ether), TAME (t-amyl methyl ether).
Another reason for using an oxygenated compound is that they burn effectively to carbon dioxide and water and there are very few other side-products, even in small amounts. With the very stringent anti-pollution legislation in many countries, oxygenated compounds are added to reduce the proportion of hydrocarbons in petrol which in turn means an overall reduction of polluting gases (carbon monoxide and unburnt hydrocarbon) in the exhaust.
Uses of methyl t-butyl ether
Methyl t-butyl ether, MTBE, is widely used as the oxygenated additive to improve the characeristics of petrol. and its production grew over 20 years from almost zero to about 16 million tonnes a year in 2002. There has now been a dramatic turnaround in the US, Canada, Japan and in Europe. It was found that even very small amounts of MTBE can contaminate drinking water. Any seepage from storage tanks in petrol stations, which is not uncommon, leads to damage to the water course, This is because MTBE is much more soluble in water than petrol and any MTBE in the ground, is carried by rainwater through the soil, whereas petrol that leaks remains close to the tank.
Many states in the US have now banned the use of MTBE in petrol. Among the first were California and New York. These two states accounted for 40% of the MTBE used for this purpose. Another 23 US states followed their lead within two years. Bioethanol (from biomass) is now the favoured oxygenate in the US, Canada and Japan in place of MTBE. Two other ethers, ETBE and TAME, are also used in the US and particularly in Europe. China and many other countries still use MTBE as their additive of choice although in China, methanol is now being used more.
Denmark in the EU was the first to reduce the use of MTBE but the amount used in the EU varies from country to country and between suppliers. For example in Finland, the proportion of MTBE in petrol is between 10 and 15%, with an average of 12%.
The petrol in the UK uses much less MTBE, one of the lowest proportions in Europe often with1-2% MTBE and a range from 0 to 10%. Apparently, the British are particularly price conscious. As MTBE is relatively costly, it is only added in significant quantities to petrol, where the high octane rating of 98 is needed. The petrol of octane rating 95, the most used, does not need an additive.
An increasing amount of ETBE (ethyl t-butyl ether) is used in Europe, particularly in France (see below). Another ether, TAME (t-amyl methyl ether) is also used in the US and Europe.
Annual production of methyl t-butyl ether
| World | 15.3 million tonnes1,2 |
| China | 5.6 million tonnes1 |
Data from:
1. For 2013, Merchant Research and Consulting, 2014
2. Predicted to be 15.2 million tonnes in 2018
Manufacture of methyl t-butyl ether
MTBE is manufactured from 2-methylpropene (isobutene) and methanol using an acid catalyst. The catalyst is an anionic ion-exchange resin and the reaction is carried out at ca 340-360 K and 8 atm pressure, with methanol in excess. The unused methanol is recovered and recycled.
.jpg)
2-methylpropene is obtained from a variety of sources:
(i) From the cracking and reforming of various fractions from the distillation of oil.
(ii) By dehydrogenation of 2-methylpropane, which is also formed as a by-product in various processes in the petrochemical industry. The vapour is passed over a catalyst (platinum and palladium on an inert support):
.jpg)
(iii) Another route from 2-methylpropane involves its oxidation to 1,1-dimethylethyl hydroperoxide (often called t-butyl hydroperoxide:
.jpg)
This process is carried out in the liquid phase, pure oxygen, under pressure, is passed through the liquid, which has been warmed to about 400 K.
Propene is then passed into liquid 1,1-dimethylethyl hydroperoxide under pressure at about 400 K with a soluble molybdenum salt as catalyst:
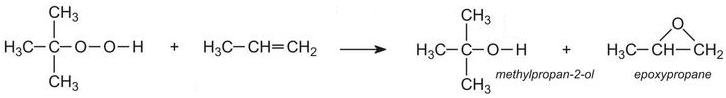
Epoxypropane (propylene oxide) is a very valuable co-product and is used, for example in the production of polyols, used to make polyurethanes.
The alcohol is dehydrated to 2-methylpropene, by passing the vapour over a catalyst:
.jpg)
Ethyl t-butyl ether (ETBE)
Ethyl t-butyl ether (ETBE) 2-ethoxy-2-methylpropane) is an alternative to MTBE as an oxygenate to enhance the octane rating of petrol. It is used mainly in Europe, particularly in France. This accounts for over 90% of the world's annual production of ca 3 million tonnes.
It is manufactured from 2-methylpropene and ethanol:
.jpg)
.jpg)
Figure 1 A plant which has been recently commissioned in Geleen in the Netherlands to produce
ethyl t-butyl ether, ETBE. The reactants are 2-methylpropene and bioethanol.
This product is referred to as bio-ETBE.
By kind permission of SABIC Europe.
It is seen as a 'greener' alternative, as bioethanol is used to make the ether.
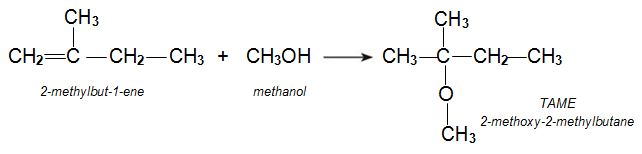
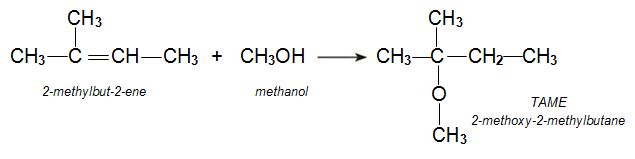
t-Amyl methyl ether (TAME)
t-Amyl methyl ether (TAME) (2-methoxy-2-methylbutane) is another alternative to MTBE. It is made from two alkenes, 2-methylbut-1-ene and 2-methylbut-2-ene produced during the various refinery processes to make petrol, including catalytic cracking.
The alkenes are mixed with methanol and passed over an ion-exchange resin such as a co-polymer of a sulfonated phenylethene and divinylbenzene. The sulfonated groups, -SO3H, provide the acidic groups which catalyse the reaction. A plug flow reactor is used.
The reaction of methanol with each of the two isomers leads to the same product, TAME:
Different countries use different oxygenates to replace tetraethyl lead as octane number improvers in petrol. Figure 2 shows the world consumption of those used in 2015.
Figure 2 World consumption of petrol octane improvers, 2015
Data from: IHS Markit, 2016
Date last amended: 31st October 2018