Soaps are made from naturally occurring animal fats and vegetable oils. The animal fats and vegetable oils are esters of the alcohol, propane-1,2,3-triol (glycerol) CH2OHCHOHCH2OH and long chain carboxylic acids (often known as fatty acids) RCO2H, where the alkyl groups contain between 7 and 21 carbon atoms.
Manufacture
The fats and oils are heated with an alkali, usually sodium hydroxide, and the esters are hydrolyzed to form a sodium salt of the carboxylic acid and the alcohol, propane-1,2,3-triol (glycerol):
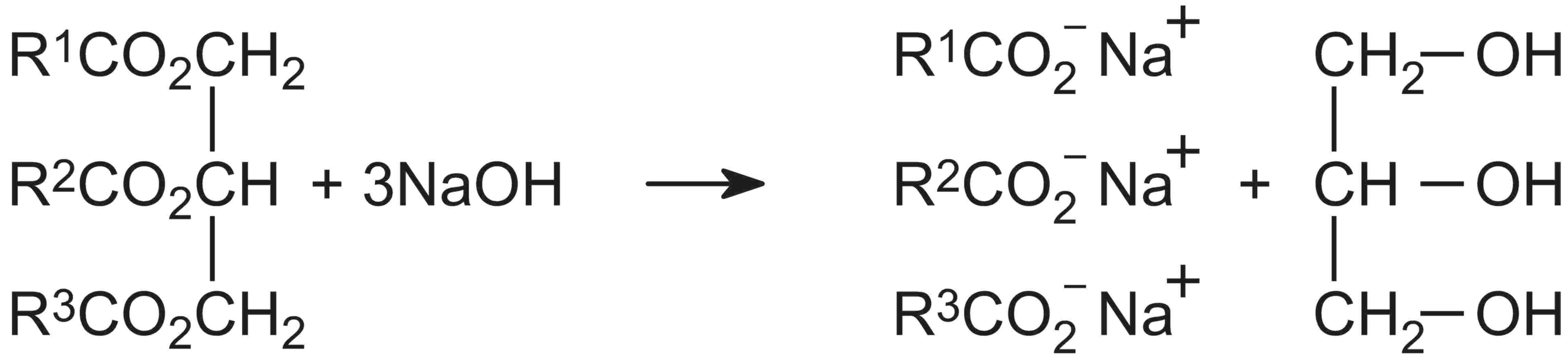
The process is known as saponification and the sodium salts of the acids are soaps.
Modern plants have continuous processes. The oils are purified, blended and then mixed with sodium hydroxide solution very rapidly. The mixture is passed into a heated reaction chamber where saponification occurs. The glycerol is more valuable than the soap and most of it is recovered. Some is left in the soap to make it smooth and soft. After saponification the soap and glycerol mixture is usually passed on to a rotating disc contact or RDC where the mixture is passed down a column and is washed with a counter-current of sodium chloride solution. Soap is not very soluble in salt water and separates out. Glycerol stays in solution which is known as lye. The washed soap is then further treated with sodium chloride solutions and centrifuged to give soap at the required concentration.
| Figure 1 Soaps are made from the sodium and potassium salts of long chain carboxylic acids. Their properties such as lathering and softness depend on the length of the carboxylic acid chain, the metal ion and the proportion of un-neutralized carboxylic acids added. They are also distinguished by their colour and perfume. Some soaps also contain germicides and exfoliates. By kind permission of Valmai Firth. |
 |
The soap is dried by spraying into a vacuum chamber to give a final product suitable for stamping.
The glycerol solution is concentrated by evaporating off the water and the glycerol is then purified by distillation. Its main use is in producing alkyd resins, used in paints. It is also used to make explosives (nitroglycerine) and in many cosmetic and pharmaceutical products.
Designing soaps
In order to give those properties to the soap the public seek, it is manufactured from a blend of animal fats (tallow) and vegetable oils (coconut and palm kernel). Some pure vegetable soaps are available, manufactured by substituting palm oil for tallow as the triglycerides have sufficiently similar composition. These are most likely to be manufactured in tropical countries where the vegetable oils are more readily available than are animal fats.
Some high quality soaps contain un-neutralised fatty acids. These help to stabilise the lather and improve the feel of the soap on the skin.
Traditional bar soaps are being increasingly replaced, particularly in developed markets, by liquid products such as shower gels, body washes and 'liquid soaps' that are formulated using synthetic surfactants rather than soaps.
Date last amended: 18th March 2013
