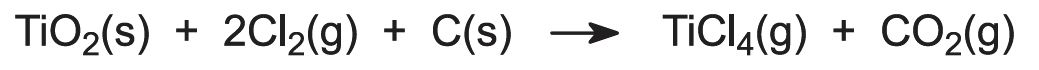Uses of titanium
Figure 1 Uses of titanium.
Almost all titanium is used as an alloy with other metals. Among the most important alloying metals are aluminium, vanadium, molybdenum, manganese, iron, tin, chromium and zirconium. One of the most used has a composition of 90% titanium, 6% aluminium, 4% vanadium which is often represented as Ti-6AI-4V.
The aerospace industry is the greatest user of titanium alloys, using them in the main airframe (body) of aircraft, and in various parts of the engines, landing gear and hydraulic tubing (Figure 2).
|
Because of their strength and resistance to chemical attack, the metal and alloys are used where other materials (even stainless steel) could rapidly deteriorate, such as in sea water. They are used in ship's propellers and in desalination plants and also in chemical plant reactors and piping.
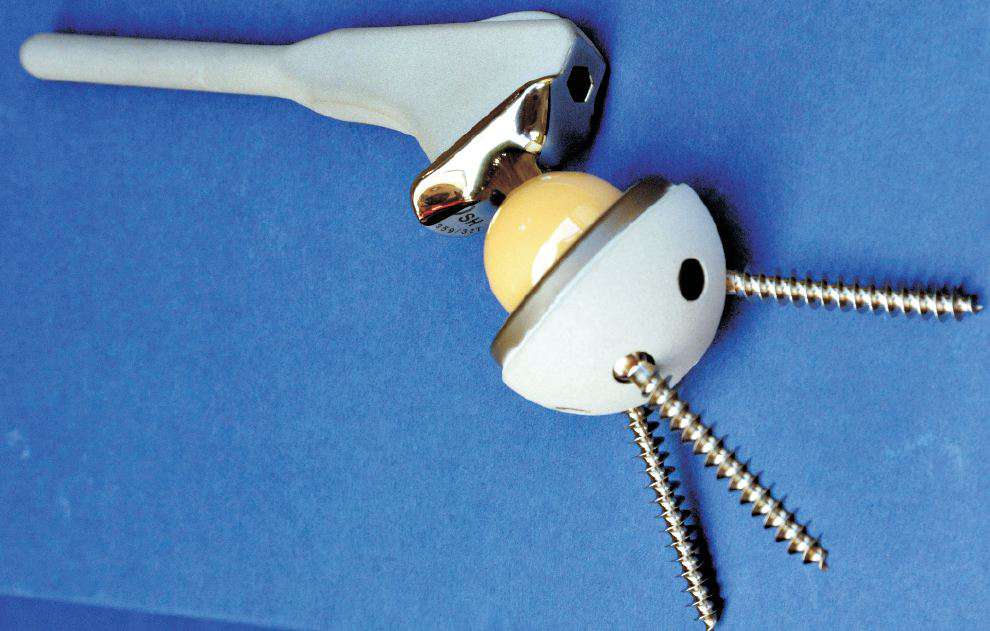
Over 1000 tonnes of titanium alloys are used annually in the world to make implants for surgery, including parts for hip and knee replacements (Figure 3). They are also used in dental implants as roots for replacement teeth, and for the cases of heart pacemakers.
|
Recently some buildings have been clad with a top surface of pure titanium sheets, to spectacular effect (Figure 4).
|
Annual production of titanium
| World | 171 000 tonnes |
| China | 80 000 tonnes |
| Russia | 42 000 tonnes |
| Japan | 30 000 tonnes |
| Kazakhstan | 9 000 tonnes |
| Ukraine | 9 000 tonnes |
Data from:
U.S. Geological Survey, Mineral Commodity Summaries, 2016.
U.S. data not available.
Manufacture of titanium
Titanium comprises 0.63% of the Earth's crust and is the fourth most abundant structural metal, after aluminium, iron and magnesium.
Titanium deposits that can be mined economically are found throughout the world. The main ores are rutile (TiO2) and ilmenite (FeTiO3) in beach sand deposits (Western Australia), ilmenite-haematite (Canada), and ilmenite-magnetite (Ukraine) in hard rock deposits (Figure 5). Although rutile is scarcer and more expensive than ilmenite, it is more commonly used because it does not contain iron compounds and can therefore be more readily processed. However, ilmenite is sometimes processed to remove the iron and make 'synthetic' rutile.
Figure 5 Stockpiling heavy mineral concentrate which contains rutile, ilmenite and
zircon, and other heavy minerals that are not valuable. It will then be further processed
to separate the rutile prior to beginning the process for the extraction of the titanium.
By kind permission of Iluka Resources.
The Kroll Process
Most titanium is manufactured from ores containing titanium dioxide using a lengthy four-stage process:
a) chlorination of the ore to titanium(IV) chloride
b) purification of titanium(IV) chloride
c) reduction of titanium(IV) chloride to titanium sponge
d) processing of titanium sponge
(a) Chlorination of the ore to titanium(IV) chloride
Titanium dioxide is thermally stable and very resistant to chemical attack. It cannot be reduced using carbon, carbon monoxide or hydrogen, and reduction by more electropositive metals is incomplete. If the oxide is converted into titanium(IV) chloride, however, a route to titanium becomes viable, as the chloride is more readily reduced.
The dry ore is fed into a chlorinator together with coke forming a fluid bed. Once the bed has been preheated, the heat of reaction with chlorine is sufficient to maintain the temperature at 1300 K:
(b) Purification of titanium(IV) chloride
The crude titanium(IV) chloride is purified by distillation, after chemical treatment with hydrogen sulfide or mineral oil to remove vanadium oxychloride, VOCl3, which boils at the same temperature as titanium(IV) chloride. The final product is pure (>99.9%) titanium(IV) chloride which can be used either to make titanium or oxidized to give titanium dioxide for pigments.
Storage tanks must be totally dry as the product undergoes rapid hydrolysis in the presence of water, generating dense white fumes of hydrogen chloride:
(c) Reduction of titanium(IV) chloride to titanium sponge
Titanium(IV) chloride is a volatile liquid. It is heated to produce a vapour which is passed into a stainless steel reactor containing molten magnesium (in excess), preheated to about 800 K in an atmosphere of argon. Exothermic reactions giving titanium(lll) and titanium(ll) chlorides cause a rapid temperature rise to about 1100 K. These chlorides undergo reduction slowly, so the temperature is raised to 1300 K to complete the reduction process. Even so, it is a lengthy process:
After 36-50 hours the reactor is removed from the furnace and allowed to cool for at least four days.
The unreacted magnesium and the chloride/titanium mixture is recovered, crushed and leached with dilute hydrochloric acid to remove magnesium chloride. In an alternative method, used in Japan, magnesium chloride, together with unreacted magnesium, is removed from the titanium by high temperature vacuum distillation.
The magnesium chloride is electrolysed to generate magnesium for the reduction stage and the chlorine is recycled for the ore chlorination stage.
The titanium is purified by high temperature vacuum distillation. The metal is in the form of a porous granule which is called sponge. This may be processed on site, or sold on to other companies for conversion to titanium products.
Figure 6 Summary of the conversion of titanium ore into useful products.
(d) Processing of titanium sponge
As titanium sponge reacts readily with nitrogen and oxygen at high temperatures, the sponge must be processed in a vacuum or an inert atmosphere such as argon. At this stage scrap titanium may be included, and other metals may be added if a titanium alloy is required. A common method is to compress the materials together to create a large block which then becomes an electrode in an electric arc melting crucible. An arc forms between the crucible and the electrode, causing the electrode to melt into the crucible where it is cooled and forms a large ingot. This may be repeated to produce a "second melt" ingot of higher quality.
ITP Armstrong Process
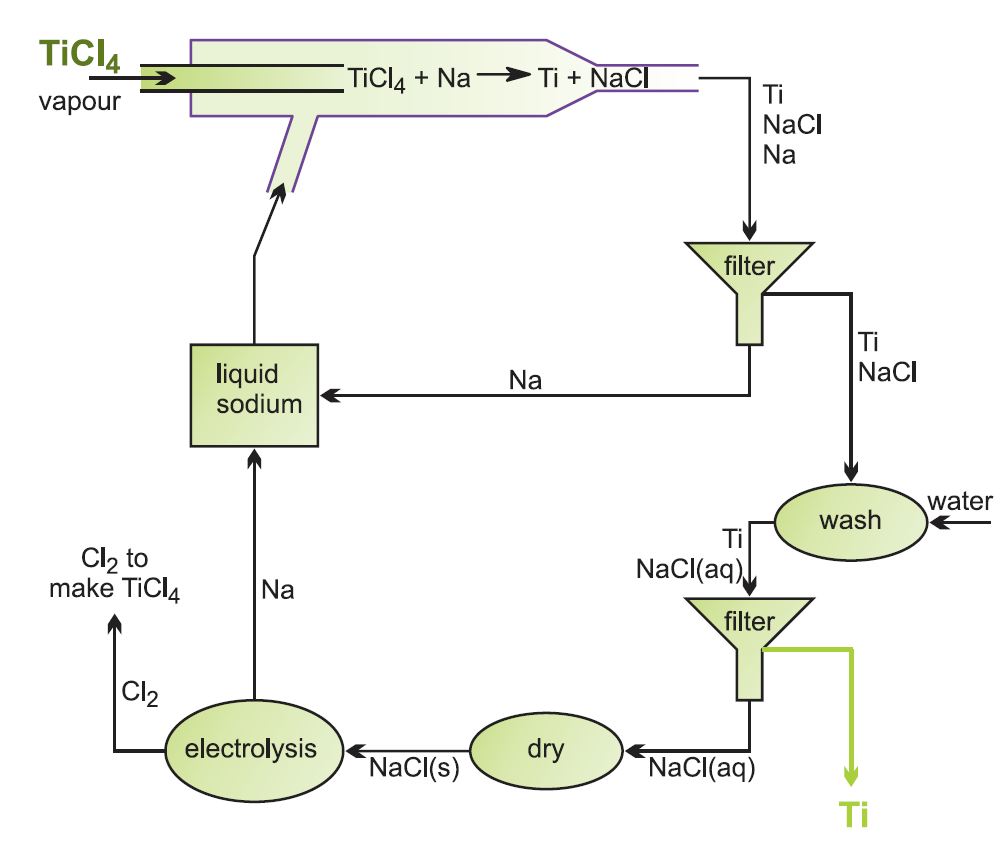
Titanium and its alloys can be produced from titanium(IV) chloride using sodium instead of magnesium. Although the chemistry is not new, a continuous rather than batch process has now been developed, significantly reducing costs.
Figure 7 A continuous process for the reduction of titanium(IV) chloride.
Titanium(IV) chloride vapour is introduced into a stream of molten sodium, and the chloride is reduced to the metal. Titanium and sodium chloride are formed as solids, and are extracted from the sodium stream by filtering. After removing residual sodium, the titanium metal can be separated from the salt by simple washing. The sodium chloride is dried, heated until molten and electrolysed, generating sodium for re-use and chlorine for the initial chlorination stage.
If the titanium(IV) chloride feed is mixed thoroughly with the correct proportions of other metal chlorides before being fed into the liquid sodium stream, the result is a very high quality titanium alloy powder, one of the major advantages of this process. For example, Ti-6Al-4V is produced by including aluminium chloride and vanadium(IV) chloride in the correct proportions in the feed.
FFC Cambridge Process
Research in Cambridge (UK) has led to the development of an electrolytic method for reducing titanium dioxide directly to titanium.
itanium dioxide (usually rutile) is powdered and then made up into pellets to act as the cathode. They are placed in a bath of molten calcium chloride and connected to a metal rod which acts as the conductor. The cell is completed with a carbon anode. On applying a voltage, titanium oxide is reduced to titanium and the oxide ions are attracted to the
carbon anode, which is oxidised to carbon monoxide and carbon dioxide (Figure 8).
Figure 8 The electrolytic reduction of titanium(IV) oxide.
If a much higher voltage is applied the mechanism is different. Calcium is deposited at the cathode and reacts with the titanium dioxide to form titanium and calcium ions are regenerated.
The process is much simpler than existing methods, operating at lower temperatures (saving energy costs), and has a lower environmental impact. It has the potential to reduce the production costs significantly, making it possible for the advantages of titanium metal to be applied to a wider range of end-products.
The process is also being considered for the production of other metals, for example, tantalum.
Date last amended: 5th October 2016