![]() Sulfur is one of the most important elements. It is used to make sulfuric acid, which is the most abundantly made industrial chemical. The element itself is an essential plant nutrient.
Sulfur is one of the most important elements. It is used to make sulfuric acid, which is the most abundantly made industrial chemical. The element itself is an essential plant nutrient.
Uses of sulfur
About 90% of sulfur produced or extracted is used to make sulfur dioxide, which is then converted to sulfuric acid. The majority of the acid is used in the production of phosphate fertilizers, a crucial component in the provision of food in the World.
Another important use of elemental sulfur is in fertilizers as a soil nutrient in its own right, particularly where there is a deficiency of sulfur in the soil.
Sulfur is beginning to be used as a component of infrastructre band structural materials. For example, adding sulfur pellets to asphalt makes road surfaces more durable and resistant to damage by cracking when the conditions are very cold. Sulfur concrete is produced by adding sulfur as concrete is being made. It has a smoother surface, making it easy to paint. It is strong and is able to withstand acidic and salt waters, making it a possible material for sea barriers and dams.
Annual production of sulfur
| World | 70 million tonnes |
| China | 11 million tonnes |
| U.S. | 9 million tonnes |
| Russia | 7 million tonnes |
| Canada | 6 million tonnes |
| Germany | 4 million tonnes |
| Japan | 3 million tonnes |
| Saudi Arabia | 3 million tonnes |
Data from:
U.S. Geological Survey, Mineral Commodity Summaries, 2016
Manufacture of sulfur
The vast majority of sulfur is recovered from natural gas and oil, which contain hydrogen sulfide and a wide variety of organic compounds that contain sulfur (such as CH3SH). They have to be removed before the natural gas and oil can be used as a feedstock for the chemical industry as they poison the catalysts that are used in the various processes to make chemicals. Equally, they have to be removed if natural gas or oil are to be used as fuels. Otherwise large amounts of sulfur dioxide, a very dangerous pollutant, will be released into the atmosphere when the fuels are burnt. The organic sulfur compounds are converted to hydrogen sulfide by reduction with hydrogen. For example:

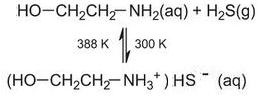
Hydrogen sulfide gas is separated from other gases present by dissolving it in an aqueous solution of an organic base, such as, for example, 2-aminoethanol (monoethanolamine). Although this is a complex process, the following equation shows the overall reaction:
 |
| Figure 1 Much natural gas and oil contain large quantities of hydrogen sulfide and organic sulfur compounds which must be removed to produce gas and oil which can be used as fuels. They are converted into elemental sulfur which is heated so that it becomes molten and then piped into large moulds where it solidifies. It is stored in the open until it is used to make sulfuric acid. This photo shows sulfur recovered in the immense Lacq field in southwestern France where the gas is very 'sour', containing 16% hydrogen sulfide. By kind permission of Arkema. |
Heating the solution of the salt liberates pure hydrogen sulfide and regenerates the amine. Hydrogen sulfide is then burned with a limited amount of air to give sulfur dioxide, sulfur and water vapour, and unreacted hydrogen sulfide:
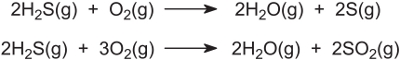
The sulfur is condensed and separated. The remaining gases are passed over a catalyst bed of alumina to convert more hydrogen sulfide to sulfur:

These two stages are repeated in three separate cycles to achieve over 95% recovery of sulfur.
Date last amended: 11th October 2016
