 Recycling of materials has become common practice over the last ten years or so, with households in many countries encouraged to save used cans, glass, plastics, paper and garden rubbish for special collection. These are then recycled for two main reasons.
Recycling of materials has become common practice over the last ten years or so, with households in many countries encouraged to save used cans, glass, plastics, paper and garden rubbish for special collection. These are then recycled for two main reasons.
One is local, to save land which would otherwise be used as dumps for the waste. In the European Union alone, 1.3 billion tonnes of waste - some 40 million tonnes of it hazardous - are thrown away annually. This amounts to about 3.5 tonnes of solid waste for every man, woman and child. On top of this there is also a further 700 million tonnes of agricultural waste to dispose of. The other main reason for recycling has global significance - to help conserve valuable resources, such as metals, wood and energy.
This unit is devoted to the recycling of metals, some basic chemicals and polymers, all within the context of the chemical industry.
Recycling of basic chemicals
Sulfuric acid
Some sulfuric acid is produced from 'spent' (used) acid and related compounds such as ammonium sulfate which is a by-product in the manufacture of methyl 2-methylpropenoate.
The acid and compounds are usually in dilute solution which is evaporated under vacuum to produce concentrated solutions. These are fed into a furnace with oxygen at about 1200 K to produce sulfur dioxide:
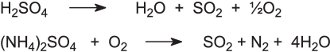
The sulfur dioxide is dried by passage through concentrated sulfuric acid. It is then oxidized to sulfur trioxide and hence sulfuric acid using the Contact Process (Unit 50).
Hydrochloric acid
The steel industry is a major user of hydrochloric acid for the pickling process to remove impurities. The industry uses a process known as pyrohydrolysis to recover the spent acid, which now contains a mixture of iron chlorides. The spent liquor is first concentrated in an evaporator, with dissolved HCl being given off and collected. The concentrated liquor is then fed into a roaster at ca 800-1000 K which converts the iron chlorides into HCl and iron(III) oxide, the HCl again being collected. For example:

HCl from both streams is absorbed in water to make 18% hydrochloric acid for reuse. It is difficult however to collect all the HCl gas, and emissions to air are a problem with this process.
Recycling within processes
Many processes recycle reactants and products in order to conserve materials and make the processes as efficient as possible. An example is in the manufacture of chloroethene (vinyl chloride), the monomer for the manufacture of PVC. Chloroethene is made from ethene via 1,2-dichloroethane, which is then cracked:
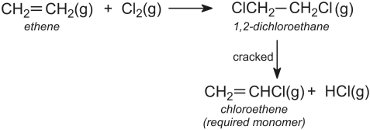
The hydrogen chloride is recycled and reacted with oxygen and more ethene. The overall reaction can be represented by:
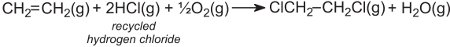
Recycling of polymers
The most written about aspect of polymers is not their enormous usefulness but the problems that they bring as waste. This is not surprising, as the world's annual production of plastics is nearly 300 million tonnes. China accounts for about 24%, and the rest of Asia another 16%, Europe 20% and NAFTA (North American Free Trade Agreement: US, Canada and Mexico) another 20%. To put these numbers in perspective, 20 000 large bottles can be made from just one tonne of plastic. Further, the plastics industry uses nearly 5% of the world's oil supply.
One of the great problems facing the industry is to ensure that the plastics can be recycled. This account is concerned with the recycling within the chemical industry, the issue of recycling all plastics produced is dealt with briefly in a later section.
Annual Production of plastics 2012
| China | 57.6 million tonnes |
| Rest of Asia | 38.1 million tonnes |
| NAFTA | 48.0 million tonnes |
| Latin America | 11.8 million tonnes |
| Europe | 49.2 million tonnes |
| Russia* | 7.2 million tonnes |
| Middle East & Africa | 17.4 million tonnes |
| Japan |
11,8 million tonnes |
*Russia plus Commonwealth Independent States that used to be part of the Soviet Union
Data from: PlasticsEurope/Consultic 2013
Reusing plastics
Reusing plastics would be ideal, and already happens for example, with bottle crates and increasingly with shopping bags. At first sight, collecting plastics which can be remoulded, for example the thermoplastics, such as poly(ethene) and poly(propene), would appear to be an attractive solution. However, collecting and sorting plastic articles into specific polymers is an expensive and difficult process. It is often done manually by trained staff who sort the plastics into polymer type and/or colour. Technology is being introduced to sort plastics automatically, using various spectroscopic techniques.
First, infrared spectrometry is used to distinguish between clear and translucent plastic. Next a vision colour sensor, programmed to ignore labels, identifies various coloured plastics (Figure 1). X-ray spectrometry is then used to detect the Cl atom in poly(chloroethene) (PVC). Finally a near infrared spectrometer is used to detect resin type, most importantly for the separation of high density poly(ethene) (HDPE) and a polyester such as PET. Typical sorting rates are of the order of 3 items per second.

Figure 1 This machine sorts bottles and other containers made from different polymers prior to cleaning and shredding. It distinguishes between different polymers using a NIR (near infra-red) detector. This distinguishes between bonds in a molecule. There is also a separate detector which allows metals to be removed.
By kind permission of S+S Inspection Ltd.
| Figure 2 The plastic bottles and containers are moving from left to right on a conveyor belt. The machine shown in Figure 1 can be seen in place and allowing the various different plastics and metals to be sorted. By kind permission of S+S Inspection Ltd. |
 |
Plastic can also be separated on the basis of density by flotation. One recently developed method involves spreading the plastic and passing it through a series of pipes in suspension in water. The flow rate of the plastic depends on the density, enabling streams to be taken off at different points along the pipe.
Recycling of polyesters, for example PET (in bottles), is now widely used. The recovered bottles are washed, ground into flakes, melted and extruded as fibres. The fibres are then used to make products such as carpets.
High density poly(ethene), HDPE, used for juice and milk bottles, is also ground into flakes, melted and pressed into sheets to be made, for example, into bin-liners or moulded into containers.
Recycling of plastic bags saves about two thirds of the energy used to produce a new bag. PVC is similarly recycled and extruded for pipes or used for window frames.
Converting polymers into monomers
Some polymers can be depolymerized to reform monomers, which can then be purified by distillation and polymerized again to produce the polymer. This still has the drawback that the polymer waste has to be sorted prior to being heated.
For example, PET waste is dissolved in the dimethyl ester of benzene-1,4-dicarboxylic acid (dimethyl terephthalic acid) and then heated with methanol under pressure at 600 K. This produces the two monomers of PET, ethane-1,2-diol and the dimethyl ester which are subsequently purified by distillation.
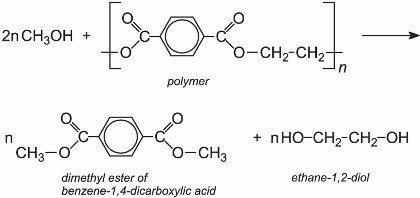
Polyamide 6 (nylon 6), used in carpets, is converted back to its monomer, caprolactam. The backing is removed from the carpet and the carpet is then shredded and pulverised. On heating, polyamide 6 depolymerizes:
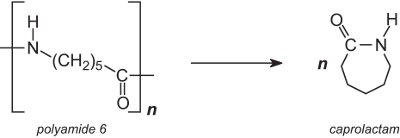
After purification, by distillation, the monomer is polymerized again to yield polyamide 6. In another process, it is not necessary to remove the backing (which is an added expense). Instead, the polyamide 6 fibres are heated in a stream of superheated steam and depolymerized.
It is less easy to reuse polyamide 6,6 which is made from two monomers. Nevertheless, DuPont has a process where carpets, with the backing removed, made from both polyamide 6 and polyamide 6,6 are shredded and pulverised before being heated strongly in an atmosphere of ammonia gas. Among the products are caprolactam (from polyamide 6) and 1,6-diaminohexane, often known as hexamethylenediamine, (from polyamide 6,6). These are purified by distillation and reused to make the polyamides.
Polyamides from recycled carpet are being used to make new carpets and to make cushions and resilient flooring. Although many recycling programmes are restricted to collecting carpets used commercially (for example in large hotels and offices), this too is about to change and domestic carpets will be used more extensively.
Cracking the polymer
Polymers, like other high molecular mass organic compounds such as the alkanes in oil, can be cracked at high temperatures to form smaller molecules. For example, if they are steam cracked, polymers such as poly(ethene) and poly(propene) yield alkenes and alkanes of small molecular mass which can be used in the same way as those formed in the cracking of naphtha. Small cracking plants are being built for this purpose.

Figure 3 This yacht is using sails made from recycled poly(ethene).
By kind permission of Shell International Ltd.
Mixtures of polymers can be converted into useful compounds either by pyrolysis or by oxidation. This has the advantage that the plastics do not have to be rigorously sorted before being treated. The mixture of polymers is heated in a stream of hydrogen at about 500 K. If the polymers contain chlorine (for example, PVC), hydrogen chloride is formed and is washed out. The remaining gases are then heated at about 700 K and cracked to form the usual mixture of hydrocarbons (alkanes, alkenes, aromatics) which can be fed into the stream of hydrocarbons formed from the steam cracking of oil fractions.
Alternatively, the gases formed from heating the mixture of polymers and following removal of the hydrogen chloride, are mixed with air and passed through a furnace at ca 1500 K to form a mixture of carbon monoxide and hydrogen, synthesis gas. This is then fed into the synthesis gas produced by the usual methods. This latter process, at present, appears to be the more favoured.
Polymers as fuels
Polymers can be burnt to produce energy. The problems are that the incineration can produce noxious fumes which must be trapped and the carbon is not recycled even if the energy can be used.
Waste management
This unit has been concerned with the recycling of plastics within the chemical industry. However, the issue of recycling plastics in a wider context is extremely important. Within Europe, 26% of plastics are recycled and another 36% are used to produce energy. This still leaves 38% (ca 10 million tonnes) to go to landfill. This contrasts with a zero target for landfill by 2020, which is being promoted within the European Union.
Packaging accounts for over 62% of the waste plastic, but over 69% of it is reused. This proportion varies very widely. Almost all is reused either by recycling or energy production in 9 European countries (Austria, Switzerland, Germany, Luxembourg, Denmark, Sweden, Belgium, Netherlands and Norway). In contrast, large countries such as Italy (72%), France (67%), Poland (64%), Spain (57%) and the UK (38%) lag behind.
Recycling of metals
The recycling of metals (often referred to as secondary production) is becoming increasingly important with more aluminium and lead being produced from recycled sources than from their ores, and vast quantities of steel and copper also being produced via recycling.
The processes are described in the units devoted to the individual metals, aluminium, copper, steel, lead and zinc.
In all cases the properties of the metals following recycling are completely unimpaired. Their quality is just the same as for metal produced from the ore.
The materials for recycling come from three sources. One is the waste material generated by the initial manufacture and processing of the metal. Another is waste material from the fabrication of the metals into products. Both of these sources are referred to as new scrap. The third, most commonly regarded by the public as recycling, is the discarded metal-based product itself (old scrap). Thus in manufacturing a car, each of the three sources of recyclable metal becomes available from the steel mill itself, from the factory making the cars and lastly when the car itself is eventually recycled.
Date last amended: 16th January 2014
