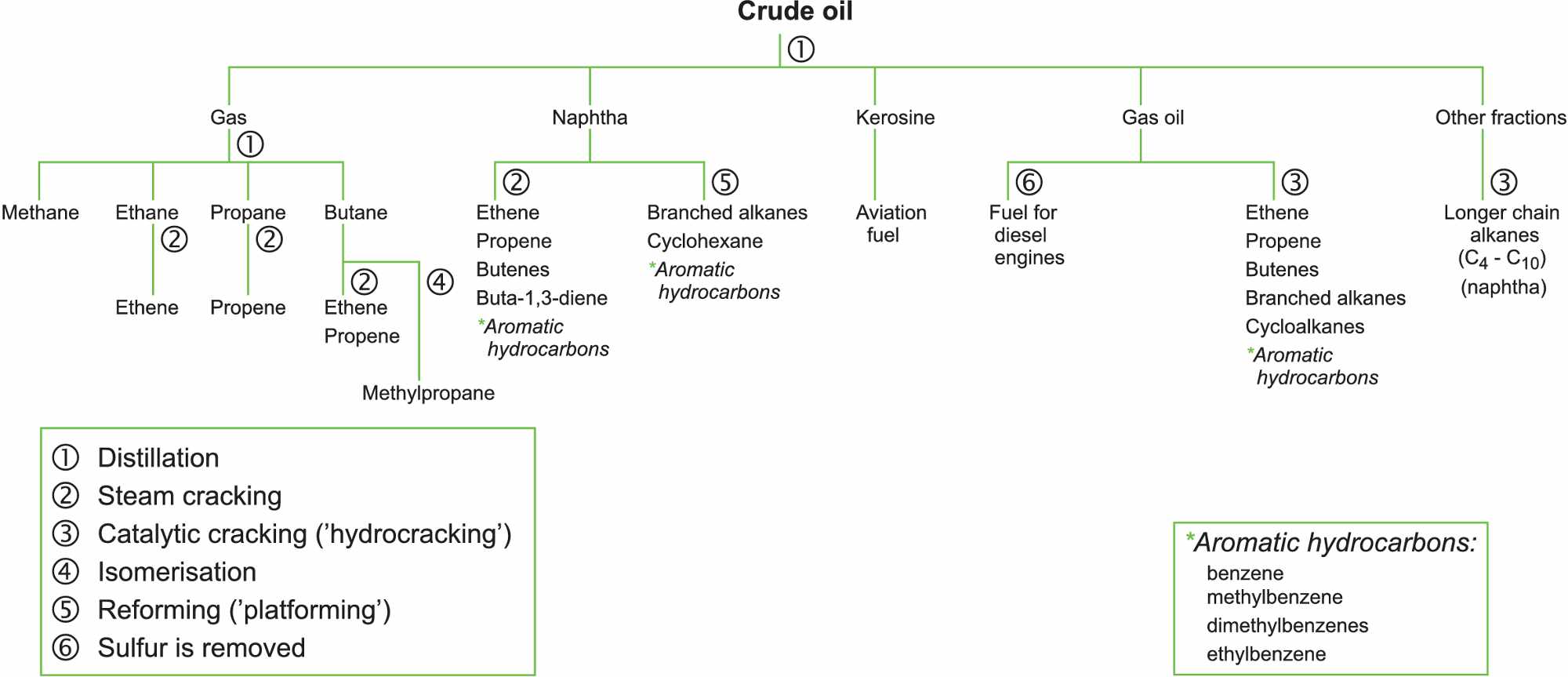
We depend largely on crude, the gases associated with it and natural gas (mainly methane) as the source of liquid fuels (petrol, diesel) and the feedstock for the chemical industry.
Oil, and the gases associated with it, consists of a mixture of hundreds of different hydrocarbons, containing any number of carbon atoms from one to over a hundred. Most of these are straight chain, saturated hydrocarbons which, except for burning, have relatively little direct use in the chemical industry or as fuel for cars.
Thus the various fractions obtained from the distillation of crude oil and the associated gases have to be treated further in oil refineries to make them useful. The most valuable fractions for the chemical industry, and for producing petrol, are liquefied petroleum gas (LPG), naphtha, kerosine and gas oil. These are treated in several ways including cracking, isomerisation and reforming.
|
The refinery
Petrol (gasoline) contains a mixture of hydrocarbons, with 5 to 10 carbon atoms. The mixture of C5-C10 hydrocarbons obtained directly from the distillation of crude oil contains a high proprtion of straight-chain alkanes. However, if this mixture is used as petrol, it does serious damage to a car's engine. Petrol containing a high proportion of straight chain alkanes tends to ignite in the cylinder of the car engine as the piston increases the pressure and before the cylinder reaches the optimum position. Ideally, the mixture of petrol vapour and air is ignited with a spark at a predetermined position of the piston in the cylinder. This problem of premature ignition is referred to as pre-ignition and also as engine knock. The term knock is used as pre-ignition can be heard. Severe knock can cause serious engine damage.
However, branched-chain alkanes, cycloalkanes and aromatic hydrocarbons are much more resistant to knock and straight-chain alkanes are converted into them in a series of processes in the refinery which are described in this unit.
The resistance of petrol to knock is measured in terms of an octane rating (octane number). The higher the number, the less likely is a fuel to pre-ignite.
The octane rating is on a scale where heptane is given an arbitary score of 0 and 2,2,4-trimethylpentane (iso-octane) one of 100. Thus a petrol with the same knocking characteristics as a mixture of 95% 2,2,4-trimethylpentane and 5% heptane has an octane rating of 95. A rating of 95 does not mean that the petrol contains just iso-octane and heptane in these proportions, but that it has the same tendency to knock as this mixture.
The octane rating of petrols usually available for cars range from 95 upwards and contain a mixture of straight-chain, branched, cyclic and aromatic hydrocarbons, produced by the processes described below.
These processes are also used to convert staight-chain hydrocarbons to hydrocarbons which are much more useful to make chemicals which are then used to make a huge range of compounds from polymers to pharmaceuticals.
Cracking
Cracking, as the name suggests, is a process in which large hydrocarbon molecules are broken down into smaller and more useful ones, for example:
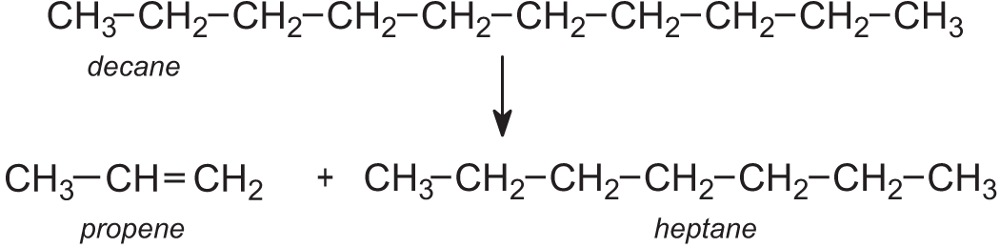
The cracking products, such as ethene, propene, buta-1,3-diene and C4 alkenes, are used to make many important chemicals. Others such as branched and cyclic alkanes are added to the gasoline fraction obtained from the distillation of crude oil to enhance the octane rating.
Cracking is conducted at high temperatures, by two processes
- Steam cracking which produces high yields of alkenes
- Catalytic cracking in which a catalyst is employed and which produces high yields of branched and cyclic alkanes
Steam cracking
Steam cracking plants (Figures 1 and 2) use a variety of feedstocks, for example
- ethane, propane and butane from natural gas
- naphtha, a mixture of C5 to C10 hydrocarbons, from the distillation of crude oil
- gas oil and residues, also from the primary distillation of oil
Very recently a cracking plant has come on stream in Singapore in which crude oil itself is the feedstock, the first time that this has been done. The advantages of this are that it cuts out the expensive distillation processes needed, for example to produce naphtha, and that it produces a wider range of products. However the disadvantage is that it may not produce the product that is needed in high enough yield. For example, if you want a high yield of ethene it is better to make it from ethane or naphtha. This disadvantage can be overcome by having more than one plant on the same site.
|
Figure 2 Naphtha is used as the feedstock to the row of furnaces on this steam cracking plant located at Wilton, UK. |
 |
Modern steam cracking plants are very large, usually producing 1-2 million tonnes of products annually and several have been built recently that can have an output of nearly 3 million tonnes a year and cost about 1 billion dollars to build,
The reactant gases (ethane, propane or butane) or the liquids (naphtha or gas-oil) are preheated and vaporised, are mixed with steam and heated to 1050-1150 K in a tubular reactor (Figure 3). They are converted to low relative molecular mass alkenes (plus by-products).
| Figure 3 Inside a tubular reactor being used for steam cracking naphtha. The temperature is about 1150 K. 1. Naphtha vapour flows through the inside of the tubes in the furnace 2 Rows of furnace guns which burn methane to generate heat inside the furnace 3 A peephole By kind permission of SABIC Europe. |
 |
The proportion of different products from steam cracking depends essentially on two factors.
(a) The composition of the feedstock
The composition of the the products depends crucially on the feedstock used. For example, a much higher proportion of ethene to other products is formed from ethane and propane than from other feedstocks. However, if more RPG (raw pyrolysis gasoline), a mixture of C5-C8 hydrocarbons, is needed, one would choose naphtha or gas oil. More details are given in Table 1.
| Product | Feedstock | |||
|---|---|---|---|---|
| Ethane | Propane | Naphtha | Gas oil | |
| Hydrogen | 5 | 2 | 1 | 1 |
| Methane | 9 | 27 | 15 | 8 |
| Ethene | 78 | 42 | 35-25 | 23-15 |
| Propene | 3 | 19 | 16 | 14 |
| Butenes | 5 | 5 | ||
| Buta-1,3-diene | 2 | 3 | 5 | 6 |
| RPG* | 3 | 7 | 19-29 | 20 |
| Fuel oil | 4 | 23-31 | ||
| *RPG (= raw pyrolysis gasoline) is a mixture of C5 - C8 hydrocarbons. RPG is selectively hydrogenated, then aromatics (benzene, methylbenzene (toluene) and dimethylbenzenes (xylenes)) are removed by solvent extraction and the residue is used as fuel, e.g. for petrol blending. | ||||
Table 1 Typical product yields (%) by mass from steam cracking
various hydrocarbon feedstocks.
Data from: Petroleum processes Volume 1, A Chauvel and G Lefebrve,
Institut Français du Pétrole Publications, 1989.
(b) The severity of the conditions used (the temperature of the reactor furnace and the time taken for the reactants to flow through it)
The conditions chosen for the furnace temperature and the flow rate of the heated reactants depend on the products that are needed, as shown in Table 2.
| Product | Low severity (1000 K residence time 0.5 s) | High severity (1150 K, residence time 0.1 s) |
|---|---|---|
| Hydrogen | 1 | 1 |
| Methane | 15 | 18 |
| Ethene | 19 | 32 |
| Propene | 16 | 13 |
| C4 hydrocarbons | 10 | 9 |
| RPG | 36 | 18 |
| Others | 3 | 9 |
Table 2 Product yields/% by mass from the steam
cracking of naphtha.
It is important to ensure that the feedstock does not crack to form carbon, which is normally formed at this temperature. This is avoided by passing the gaseous feedstock very quickly and at very low pressure through the pipes which run through the furnace. There is however, a problem; if the plant is run at sub-atmospheric pressure, there may be a leak that allows air to enter into the gases and form an explosive mixture. This is prevented by mixing the feedstock with steam. The steam also acts as a diluent and inhibits carbonisation.
This endothermic reaction occurs in less than a second as the hydrocarbon mixture passes through tubes within the radiant section of the cracking furnace. The products are cooled rapidly (quenched) to prevent loss via side reactions and separated in a series of processes including compression, absorption, drying, refrigeration, fractionation and selective hydrogenation.
 |
|
Figure 4 A view of the steam cracking unit at Wilton in the north-east of England. The products from steam cracking include a mixture of C1 - C4 hydrocarbons and are separated by fractional distillation. Some of the columns are: |
A steam cracker is one of the most technically complex and energy intensive plants in the chemical industry. It has equipment operating from 100 K to 1400 K and near vacuum to 100 atm. Whilst the fundamentals of the process have not changed in recent decades, improvements continue to be made to the energy efficiency of the furnace, ensuring that the cost of production is continually reduced.
Catalytic Cracking
A catalyst allows lower reaction temperatures to be used. In fluidised catalytic cracking, the feedstock is gas oil which is vaporised and passed through a zeolite, produced as a fine powder (Unit 2), heated to about 700-800 K in the reactor. It is so fine that it behaves like a fluid and continuously flows out of the furnace with the cracking products. The temperature, residence time and the catalyst determine the product proportions. After cracking, the catalyst is separated from the products, regenerated by burning off deposited carbon in air (900 K), and subsequently recycled.
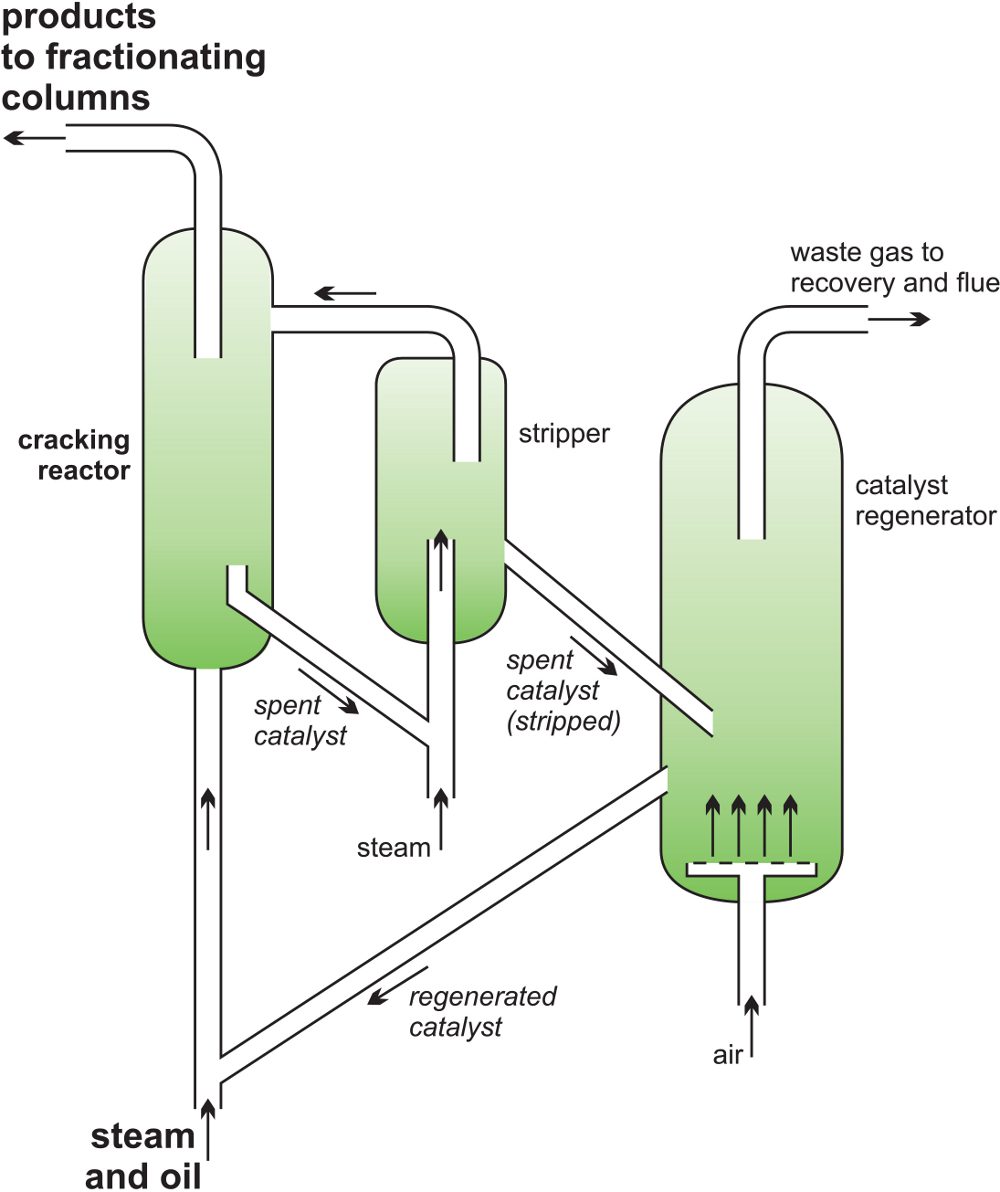
Figure 5 A catalytic cracker as used to produce alkenes from gas oil.
The products are:
- a gas of which ethene and propene are the main constituents
- a liquid which is used for petrol and contains branched- chain alkanes, cycloalkanes and aromatic hydrocarbons
- a high boiling residue used as a fuel oil
The relative proportions of the products, as noted above, can be altered by changing the catalyst and temperature. One of several zeolites can be used. For example, if the chosen zeolite contains ZSM-5, the propene yield is increased.
A variant of the process is known as hydrocracking. The cracking is carried out with hydrogen at a pressure of 80 atm and a catalyst of finely divided platinum on silica or alumina. Because excess hydrogen is present no alkenes are formed, and high proportions of branched alkanes, cycloalkanes and aromatics are produced which are essential in the formulation of high grade 'green' petrol. The hydrogen also decreases the tendency for the hydrocarbons to form finely divided carbon on the catalyst surface. The reaction products are separated by fractionation.
Hydrocracking is also used to crack heavy gas oils (which have over 20 carbon atoms in the hydrocarbon molecule) to shorter chain molecules similar to those in naphtha, which can then be steam cracked to form alkenes.
Isomerisation
Isomerisation is the process in which hydrocarbon molecules are rearranged into a more useful isomer. For example:
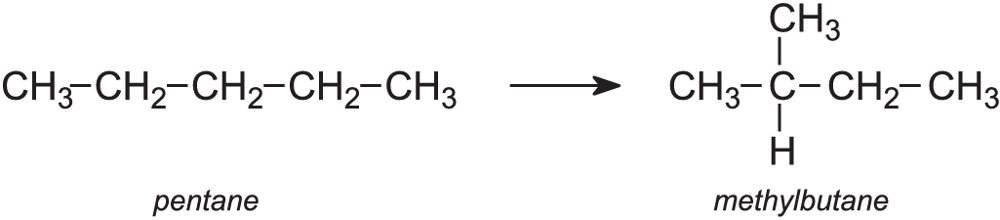
The process is particularly useful in enhancing the octane rating of petrol, as branched alkanes burn more efficiently in a car engine than straight-chain alkanes.
An important example is the isomerisation of butane (from LPG) to 2-methylpropane (isobutane):
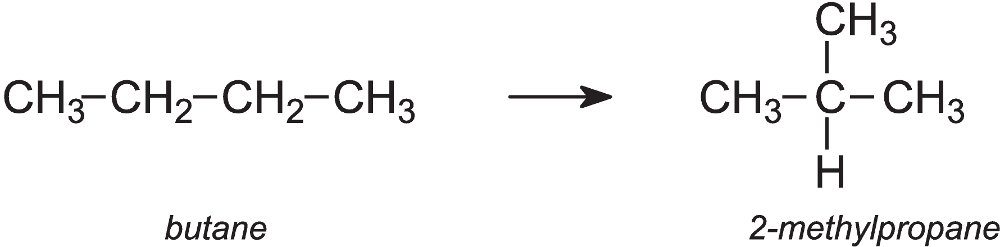
Butane vapour is passed over a solid catalyst, aluminium chloride, on an inert solid at ca 300 K. The two alkanes are then separated either by distillation or by passing them through a molecular sieve, an aluminosilicate. The branched chain alkane is trapped and the straight chain passes through and is recirculated into the reactor. The 2-methylpropane is subsequently released and used to make a branched alkane, 2,2,4-trimethylpentane (iso-octane), for petrol.
Reforming
Reforming is another process in which hydrocarbon molecules are rearranged into other molecules, usually with the loss of a small molecule such as hydrogen. An example is the conversion of an alkane molecule into a cycloalkane or an aromatic hydrocarbon for example:
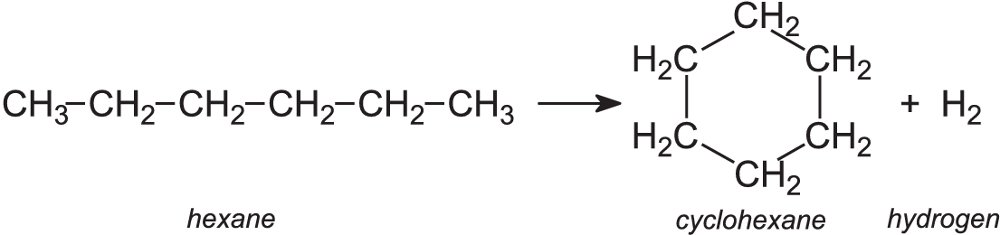
This is a very important process for the petroleum and chemical industries. It enables straight chain alkanes to be converted into branched-chain alkanes, cyclohexanes and aromatic hydrocarbons which are used to enhance the octane number of petrol.
 |
Figure 6 A view of three fixed bed reactors (Unit 3) which operate in series at an oil refinery in Fawley on the south coast of England. The reforming reaction which produces aromatic hydrocarbons and hydrogen takes place in reactor 1, followed by isomerisation reactions in reactor 2 and finally in reactor 3 cracking reactions. It also provides the chemical industry with very important compounds such as benzene, methylbenzene, the dimethylbenzenes, ethylbenzene and cyclohexane which are key materials for the polymer and other industries. By kind permission of ExxonMobil. |
The alkanes, usually the naphtha fraction, are mixed with hydrogen and passed over a catalyst at ca 700 K and a pressure of ca 30 atm. The catalyst is generally platinum or rhenium dispersed on alumina. Because platinum is involved, the reforming is sometimes called platforming. The hydrogen ensures that the resulting alkenes and cycloalkenes subsequently react with hydrogen to form saturated compounds.
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. In a refinery, alkylation refers to the alkylation of alkanes, for example, 2-methylpropane (isobutane) with alkenes, in the presence of a strong acid catalyst such as hydrofluoric acid or sulfuric acid. The reaction is carried out at mild temperatures (between 273 and 303K). Cooling is needed as the reaction is exothermic.
The product from 2-methylpropane and 2-methylpropene (isobutene) is a mixture of, branched-chain alkanes, mainly 2,2,4-trimethylpentane (isooctane):
.jpg)
With propene, 2-methylpropane forms a mixture containing a high proportion of 2,3- and 2,4-dimentylpentanes. These mixtures have very good antiknock properties and are added to petrol to increase the octane rating.
If sulfuric acid is used as the catalyst. many refineries will have a dedicated plant which takes in the waste sulfuric acid from the alkylation plant. In the recycling of sulfuric acid, the diluted acid is heated strongly to form sulfur dioxide which is then fed into a contact process plant, regenerating pure acid.
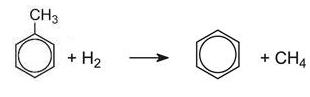
Dealkylation
The opposite of alkylation occurs when methylbenzene is heated with hydrogen over a catalyst. The dealkylation of methylbenzene, produces benzene, a more valuable product in the chemical industry. Methylbenzene vapour and hydrogen are passed over a catalyst of chromium, platinum or molybdenum, supported on silica or aluminium oxide at 820-920 K at 40-60 atm pressure:
Disproportionation
Disproportionation is said to occur, when a reactant is transformed into two or more dissimilar products. For example, methylbenzene is converted by disproportionation to more valuable products in the chemical industry, benzene and the dimethylbenzenes:
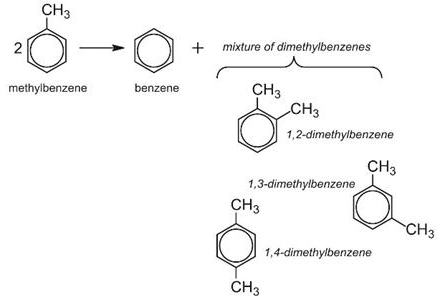
Methylbenzene and hydrogen are passed over a zeolite catalyst at 15-25 atm and 700-750 K. Hydrogen is not itself consumed in this process, but suppresses undesirable side reactions and facilitates the transfer of the methyl group. Today, by using specific zeolite catalysts, the process is highly selective towards 1,4-dimethylbenzene.
Polymerization
When many molecules of a simple compound join together, the product is called a polymer and the process is termed polymerization. The simple compounds are generally produced in the refinery and the polymers, such as poly(ethene) and poly(propene) are often manufactured nearby.
Small polymers, known as oligomers, are also produced from the refinery products. One important example is the production of alkenes with 10-14 carbon atoms from ethene, used in the manufacture of alkyl sulfonates, an important surfactant.
Date last amended: 7th September 2014

