.jpg) Benzene is the fundamental building block of aromatic compounds. It, and the methylbenzenes (toluene and the xylenes), are manufactured from fractions obtained from the distillation of oil and are used as intermediates in the production of a very wide range of chemicals as well as in petrol. They are amongst the most important organic chemicals.
Benzene is the fundamental building block of aromatic compounds. It, and the methylbenzenes (toluene and the xylenes), are manufactured from fractions obtained from the distillation of oil and are used as intermediates in the production of a very wide range of chemicals as well as in petrol. They are amongst the most important organic chemicals.
Uses of benzene and methylbenzenes
Benzene
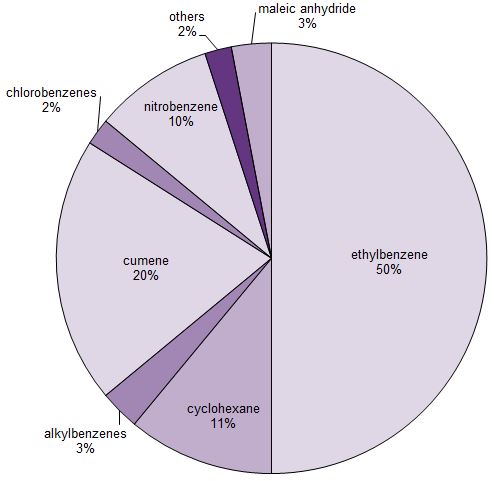
About half of the benzene that is manufactured is used to produce ethylbenzene, which in turn is used to make poly(phenylethene) (polystyrene). (1-methylethyl)benzene (cumene) accounts for another quarter, which is used to make phenol and propanone (acetone) which are converted, for example, into a wide range of polymers. Another important use is to make cyclohexane, which is used, in turn, to make hexanedioic acid (adipic acid) and caprolactam, essential intermediates in the manufacture of polyamides.
Figure 1 Uses of benzene.
From J S Plotkin, Benzene's Unusual Supply-Demand Dilemma, American Chemical Society, 2015
Nitrobenzene (Figure 1) is reduced to phenylamime (aniline) used to make azo dyes, methylene diphenyl diisocyanate (MDI) used to make polyurethanes and it is also used in the manufacture of the analgesic, paracetamol. Another use of benzene is in the manufacture of alkylbenzene sulfonates, important surfactants.
Methylbenzene (Toluene)
Over 50% of the methylbenzene (toluene) produced in the refinery is converted into benzene by dealkylation and disproportionation.
Methylbenzene is also used to make TDI (toluene diisocyanate or methylbenzene diisocyanate), one of the reagents used to make polyurethanes. It is also widely used as solvent, for example, for alkyd polymers (resins) used in paints.
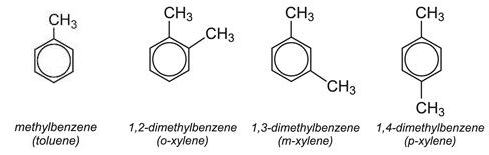
Dimethylbenzenes (Xylenes)
The most widely used dimethylbenzene is 1,4-dimethylbenzene (p-xylene). It is oxidised to benzene-1,4-dicarboxylic acid (terephthalic acid) which reacts with ethane-1,2-diol (ethylene glycol) to form the monomer for the polyester, polyethylene terephthalate (PET).
1,2-Dimethylbenzene (o-xylene) is oxidised to benzene-1,2-dicarboxylic anhydride (phthalic anhydride) which is in turn converted into the esters of benzene-1,2-dicarboxylic acid, for example di-octylbenzene-1,2-dicarboxylate, which are used as plasticisers. It is also used to make alkyd resins.
Annual production of benzene and methylbenzenes
Benzene
| World | 46 million tonnes1 |
| China | 11.9 million tonnes2 |
| Japan | 9.5 million tonnes2 |
| South Korea | 6.9 million tonnes2 |
| Europe | 6.8 million tonnes3 |
| U.S. | 3.8 million tonnes1 |
| Germany | 2.4 million tonnes2 |
1,4-Dimethylbenzene4
| World | 38 million tonnes5 |
| U.S. | 3.8 million tonnes5 |
Data from:
1. J S Plotkin, Benzene's Unusual Supply-Demand Dilemma, American Chemical Society, 2015
2. Estimated from Merchant Consulting and Research Group, 2014
3. APPE (Association of Petroleum Producers in Europe), data for 2015
4. Data are often given for the mixed isomers. It is estimated that 81% of the mixture is the 1,4 isomer, IHS Markit, 2015
5. Predicted for 2015, Argus DeWitt, 3013
Manufacture of benzene and methylbenzenes
Over the last 50 years, the vast majority of benzene, the methylbenzenes and ethylbenzene have been manufactured from the cracking and reforming of fractions obtained from the distillation of oil.
 |
| Figure 2 Benzene and the methylbenzenes are produced by a variety of ways, including cracking, reforming and isomerisation. This photo shows a plant where these methods are used and also how the aromatic hydrocarbons are purified. 1 A reactor in which the C6-C8 stream is being dehydrogenated to form aromatic hydrocarbons, including benzene and the methylbenzenes, an example of reforming. 2 A reactor in which isomerisation reactions are taking place, for example to form branched chain alkanes from naphtha. 3 A reactor in which cracking is taking place, long chain alkanes being cracked to smaller alkanes and alkenes. 4 Separation of hydrogen and C1-C3 hydrocarbons from higher hydrocarbons. 5 Removal of sulphur-containing compounds. 6 A distillation column separating C5-C6 from C7-C9 hydrocarbons. 7 A stack to remove combustion gases from the furnace. |
Today, benzene and the methylbenzenes are primarily produced via:
a) steam cracking of naphtha and other liquid feeds
b) catalytic reforming of naphtha
These two processes provide, in equal shares, about 80% of the benzene produced. In the US, this is split 30% and 50% respectively but the proportion depends on many factors such as the use made of the other products.
Smaller amounts of benzene, in total about 20%, are produced from methylbenzene via:
c) dealkylation
d) disproportionation
In the US most of this benzene is produced by disproportionation.
e) An increasing amount of benzene is being made from biomass
(a) Steam cracking of naphtha to produce benzene and dimethylbenzenes (xylenes)
This process is a major source of alkenes (ethene and propene), key 'building blocks' for the chemical industry. A valuable co-product is RPG (raw pyrolysis gas), a liquid rich in aromatic hydrocarbons (benzene, methylbenzene (toluene) and the dimethylbenzenes (xylenes)). This mixture of aromatic hydrocarbons is known as BTX. Steam cracking of alkanes such as propane and butane obtained in the distillation of oil also yields BTX.
To obtain these aromatic compounds, they are separated from the non-aromatic compounds by solvent extraction (for example, using diethylene glycol (3-oxa-pentane-1,5-diol)) and are then fractionally distilled into benzene, methylbenzene and the dimethylbenzenes.
The relative proportion of the different aromatic hydrocarbons obtained from steam cracking depends on the conditions employed. A typical composition is benzene ca 50%, methylbenzene can vary between 3 and 30% and the dimethylbenzenes are usually ca 20%.
(b) Catalytic reforming of naphtha to produce benzene and dimethylbenzenes (xylenes)
Reforming of naphtha produces petrol with a high octane rating. It is reformed using platinum or rhenium, dispersed on alumina as the catalyst. The product contains BTX.
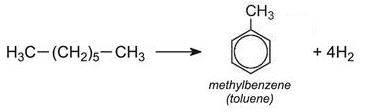
For example hexane is reformed to benzene:
.jpg)
Similarly, methylbenzene and dimethylbenzenes are formed from heptane and octane respectively:
.jpg)
As above, they are separated from the non-aromatic compounds by solvent extraction (for example, using diethylene glycol (3-oxa-pentane-1,5-diol)) and are then fractionally distilled into benzene, methylbenzene and the dimethylbenzenes.
A typical composition of BTX obtained by reforming is benzene ca 15%, methylbenzene ca 40% and dimethylbenzenes ca 45%.
Most countries use steam cracking and catalytic reforming to obtain benzene, the proportion of each process depending on whether the main aim is to produce alkenes (ethene, propene) or high grade petrol (which needs branched alkanes, cyclohexanes and the methylbenzenes) or specific aromatic hydrocarbons.
However, a major disadvantage is that there is not sufficient demand for the methylbenzene produced but it can be further treated to make the more useful benzene (see (c) and (d) below).
.jpg) |
Figure 3 This is part of BP's refinery at Castellon near Valencia, in Spain. This photograph shows the catalytic reforming unit in which benzene and other aromatic hydrocarbons are produced.
|
(c) The dealkylation of methylbenzene (toluene) to produce benzene
The dealkylation of methylbenzene, obtained from one of the above processes, produces benzene.
Methylbenzene vapour and hydrogen are passed over a catalyst of chromium, platinum or molybdenum oxides, supported on silica or aluminium oxide at 750-870 K at 40-60 atm pressure:
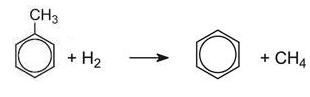
Because hydrogen is used, the process is often termed hydrodealkylation (HDA).
(d) Disproportionation of methylbenzene (toluene) to produce benzene and dimethylbenzenes (xylenes)
Benzene is the fundamental building block of aromatic compounds. It, and the methylbenzenes, are manufactured from fractions obtained from the distillation of oil and are used as intermediates in the production of a very wide range of chemicals as well as in petrol. They are amongst the most important organic chemicals.
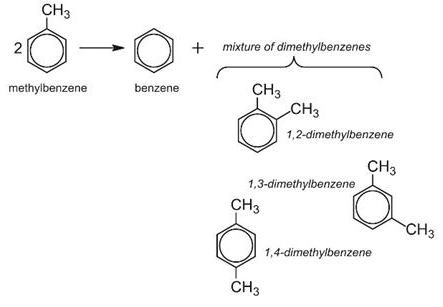
In this process, methylbenzene and hydrogen are passed over a zeolite catalyst at 15-25 atm and 700-750 K. Hydrogen is not itself consumed in this process, but suppresses undesirable side reactions and facilitates the transfer of the methyl group.
Alas, the mixture produced only contains about 25% 1,4-dimethylbenzene, the isomer that is most needed in industry, particularly for the manufacture of the polyesters. The others, 1,2- and 1,3-dimethylbenzenes, are only needed in comparatively small quantities.
However, if the zeolite is washed with phosphoric acid and heated strongly, minute particles of phosphorus(V) oxide are deposited on the surface making the pores slightly smaller. This restricts the diffusion of the 1,2- and 1,3-isomers and they are held in the pores until they are converted into the 1,4-isomer and can escape (Figure 4).
This remarkable selectivity enables the yield of the 1,4-isomer to be increased from 25% to 97%.
(e) From biomass
There is considerable interest in producing benzene and other aromatic hydrocarbons from biomass. There are several promising avenues:
- By heating finely divided biomass, such as sawdust, which can act as a fluid as it passes over a heated zeolite catalyst in the absence of air to form a wide range of compounds from which aromatic hydrocarbons can be distilled out.
- By gasification of biomass to form an oil, known as bio-oil, which is rich in aromatic hydrocarbons.
- By bioforming.
- 1,4-Dimethylbenzene (para-xylene) can be produced from bio-based ethene.
Date last amended: 18th October 2016