Distillation is used to separate mixtures of liquids by exploiting differences in the boiling points of the different components.
The technique is widely used in industry, for example in the manufacture and purification of nitrogen, oxygen and the rare gases. However, one of its best known uses is the refining of crude oil into its main fractions, including naphtha, kerosine and gas oil. This is described below. This is the first stage of converting oil into compounds which are then used to manufacture everything from plastics to medicines.

Figure 1 The steam cracking plant at Carling (near Metz in Eastern France) is dominated by the
distillation towers. The plant produces ethene and propene which are converted on site
to poly(ethene) and poly(propene).
By kind permission of Total.
Distillation of crude oil
Crude oil is a mixture of many hundreds of liquid hydrocarbons. Dissolved in it are many other hydrocarbons some of which are solids and some gases (the lower members of the alkane family, predominantly methane and ethane but often with some propane and butane). There may also be some hydrocarbon gases trapped above the oil, as for example in some of the oil fields in the North Sea.
In the refineries the oil is distilled into liquid fractions with different boiling point ranges which are then further processed.
The crude oil is heated in a furnace (ca 650 K) and the resulting mixture fed as a vapour into a fractionating tower which can have a height of 25-100 m, handling volumes of over 40 000 m3 a day. The column may contain 40-50 steel ‘sieve trays’ which fit horizontally across the column and are designed to ensure there is intimate mixing between the descending liquid, formed by condensation, and the rising vapour. To effect this close contact, the trays have holes in them (‘the sieve’) through which the vapour flows up into the liquid collecting on the trays (Figure 2).
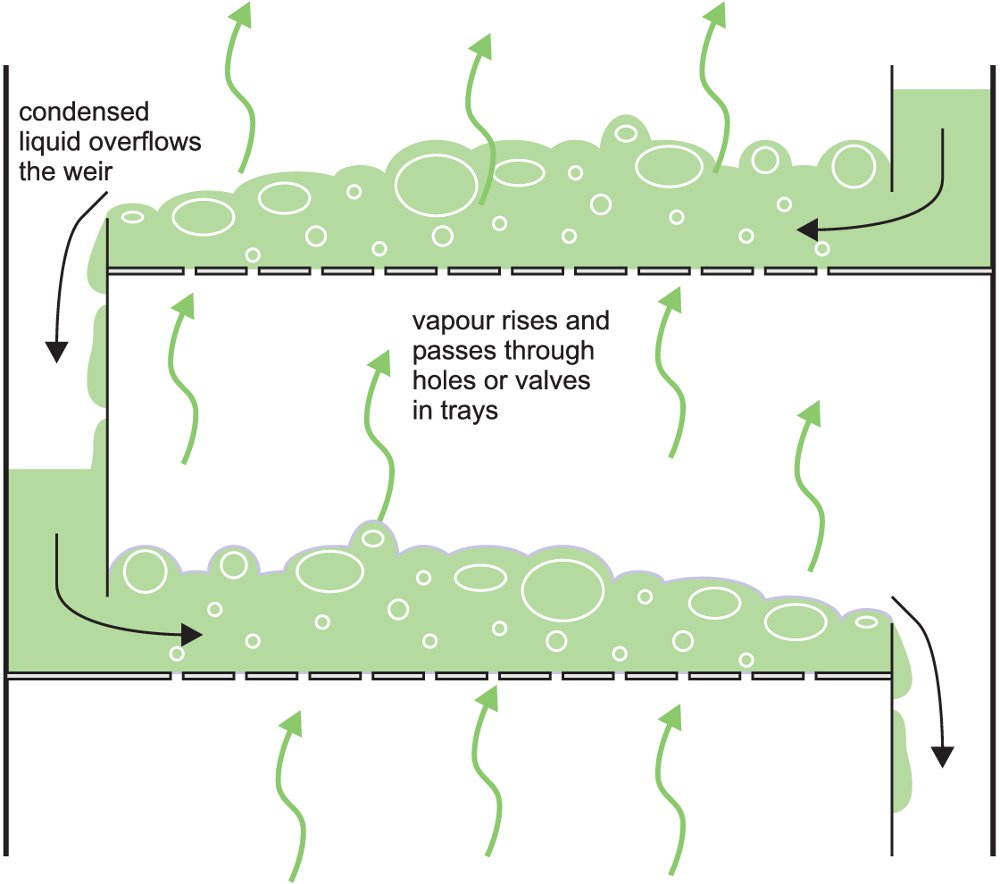
Figure 2 Trays in a fractionating column.
Alternatively, there may be valves fitting over the holes, which lift up when the pressure of the vapour below the tray is greater than the pressure on the tray (Figure 7). These are considered to be more efficient in fractionation than sieve trays without valves.
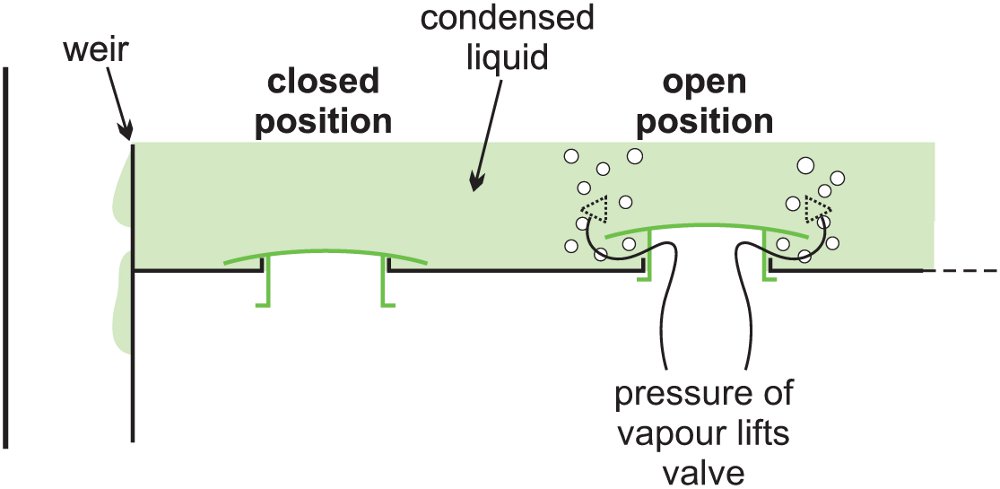
Figure 3 Valve trays.
A temperature gradient exists in the tower, the top being cooler than the bottom. When the rising vapour reaches a tray containing liquid whose temperature is below the boiling point (bp) of the vapour, it partially condenses. As some of the vapour condenses to a liquid, the dissipated latent heat then heats more liquid, and the more volatile components in the liquid evaporate joining the remaining vapour and passing up the tower. The less volatile liquid flows across the tray and down a pipe to the tray below.
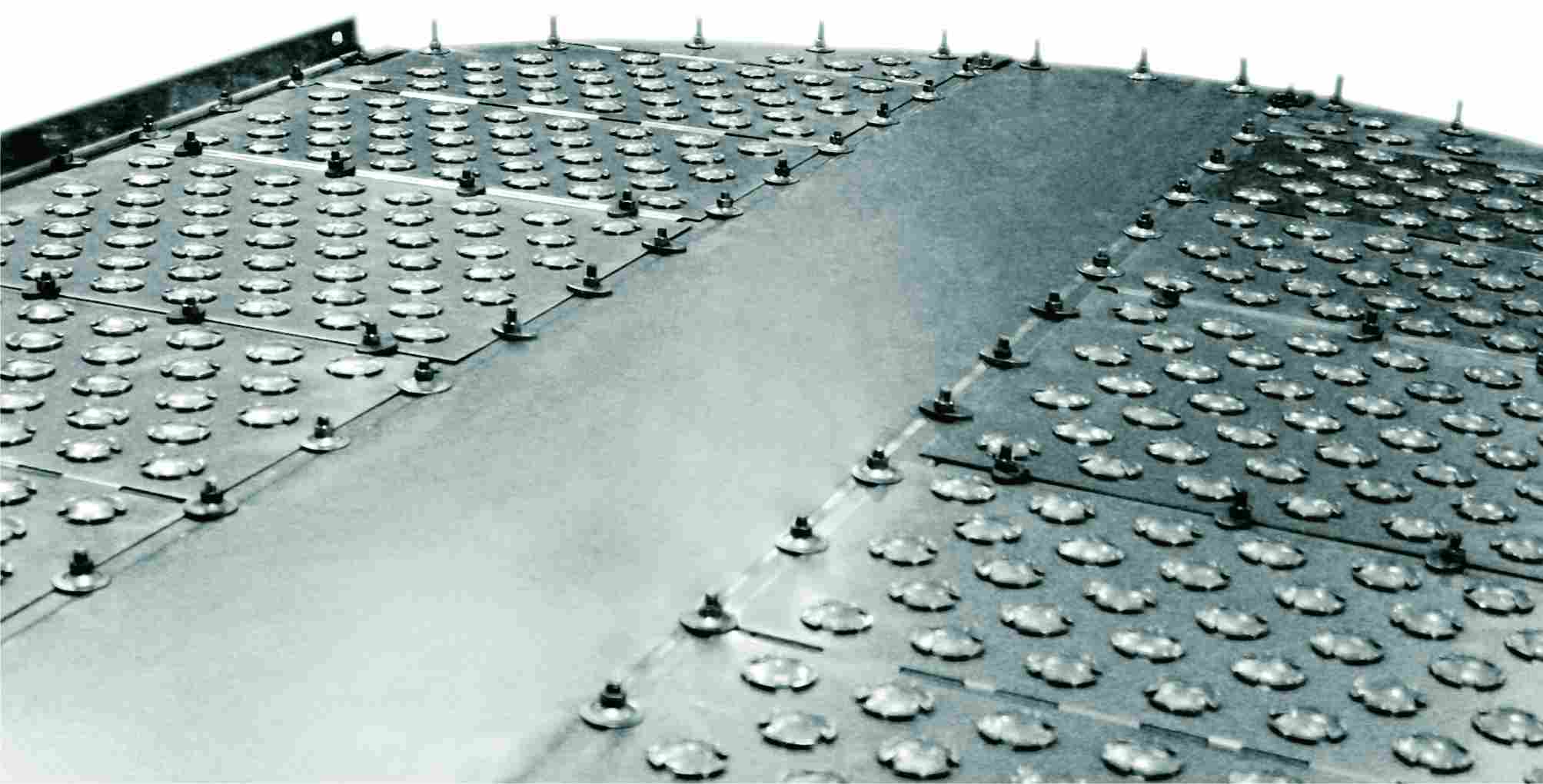
Figure 4 This FLEXITRAYTM valve tray is a steel sheet on which liftable valves are mounted.
They are much more efficient than sieve trays.
By kind permission of Koch-Glitsch, LP.
This process occurs continuously in each tray, the least volatile vapour components condensing and the most volatile evaporating. This results in each tray containing products with a comparatively narrow boiling point range (called a ‘close cut’ of products). This leads to the low relative molecular mass products (low bp) accumulating near the top of the tower and high relative molecular mass constituents (high bp) collecting near the bottom (Figure 5).
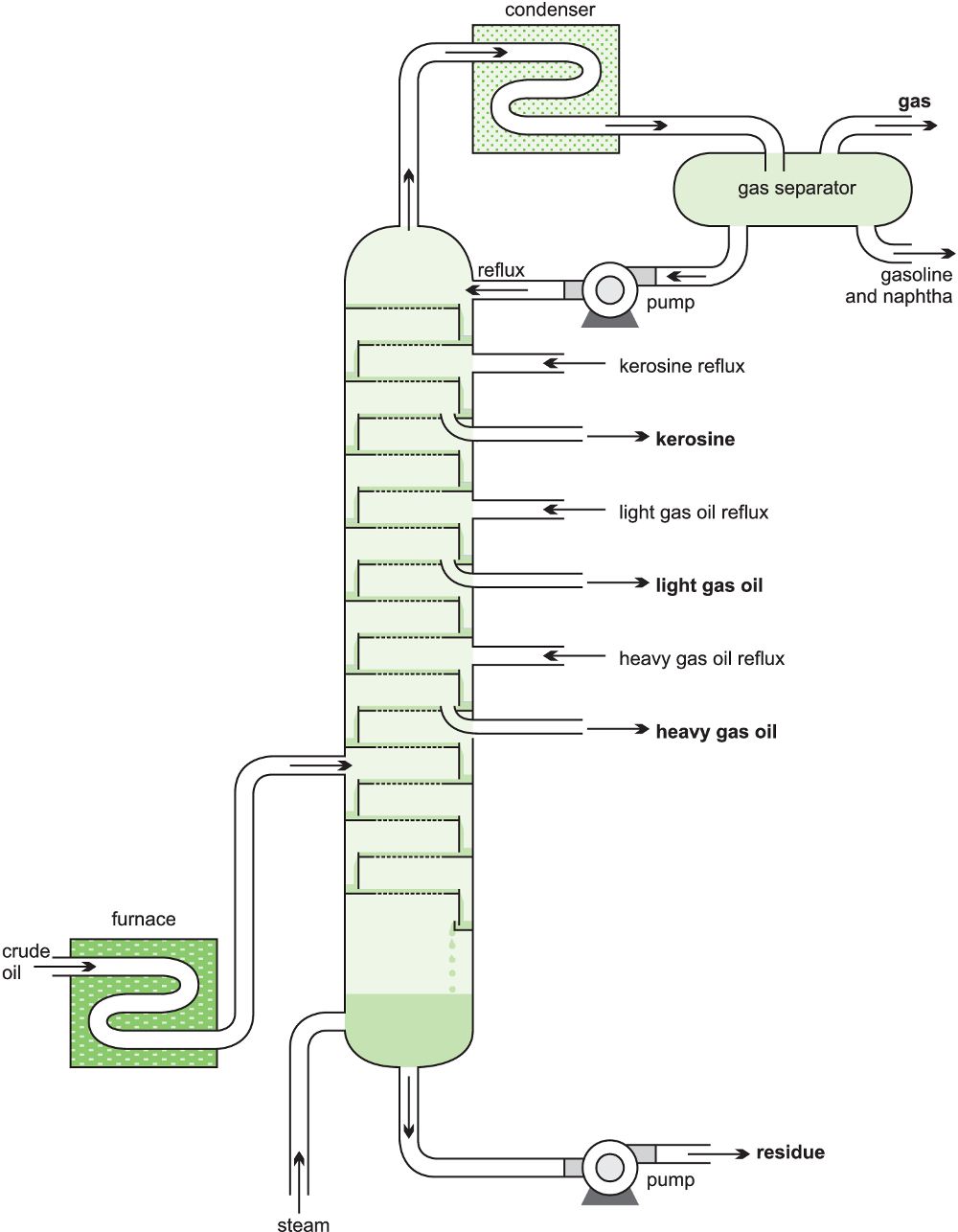
Figure 9 The fractional distillation of crude oil.
The high boiling point residue from the crude oil is then transferred to another column and distilled under vacuum; lowering the pressure reduces the boiling point and ensures constituents distil at temperatures below their decomposition temperature. From this process, lubricating oils and waxes are obtained. The final residue from the process is bitumen. Details of the fractionation are given in Table 1. The constituents in crude oil vary from one oil field to another (for example, the proportion of naphtha from North Sea oil is considerably greater than that from Middle East oil) so the numbers given are approximate.
Date last amended: 14th March 2016

