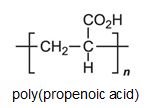
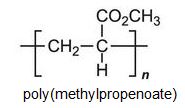
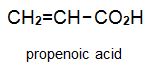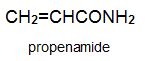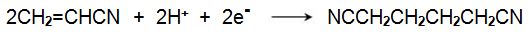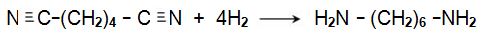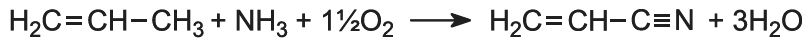There is a group of polymers, the acrylics, which can be regarded as based on acrylic acid, more formally named propenoic acid.
The acid polymerizes by addition polymerization to poly(propenoic acid) (polyacrylic acid).
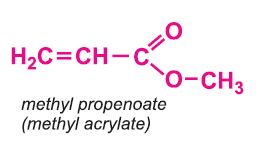
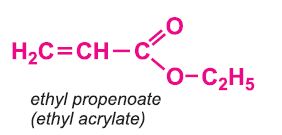
Other compounds, based on the acid, which form acrylics include the methyl, ethyl and butyl esters of propenoic acid.
 |
 |
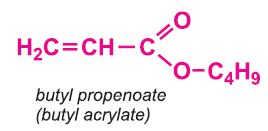 |
Each of these esters can be polymerized, for example the methyl ester forms this acrylic:
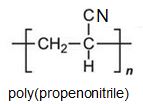
Two other important monomers, based on the acid, are propenonitrile (acrylonitrile) and propenamide (acrylamide).
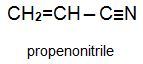 |
|
It can be seen that the polymers have the basic structure of the acrylics shown above.
 |
|
Poly(methyl 2-methylpropenoate, (poly(methyl methacrylate)), is another important polymer and is known simply as acrylic or acrylic glass. It is based on the methyl ester 2-methylpropeonic acid (methacrylic acid).
This website contains three units on the acrylics:
Poly(propenoic acid) (Polyacrylic acid)
Poly(propenonitrile) (Polyacrylonitrile)
Poly(methyl 2-methylpropenoate) (Poly(methyl methacrylate))
This unit is concerned with propenonitrile (acrylonitrile) and the polymers produced from it.
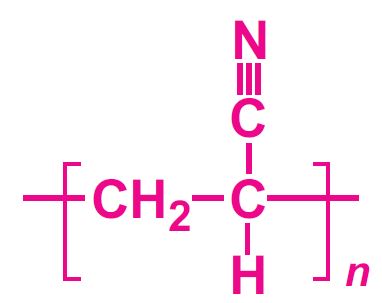
Propenonitrile is thus itself an important industrial chemical that is used in the production of a range of polymer products.
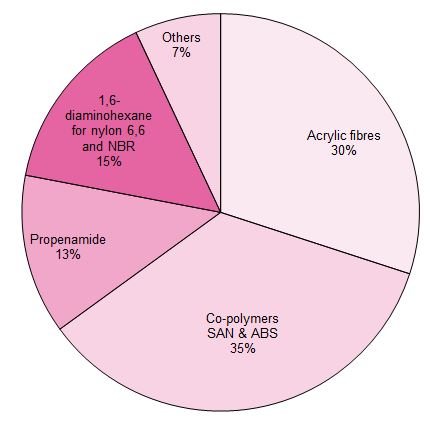
Uses of propenonitrile (acrylonitrile)
Propenonitrile is used almost entirely in the polymer industry.
Figure 1 Uses of propenonitrile
Its main uses are:
To make fibres.
These are co-polymers produced by the polymerization of propenonitrile and another monomer, often either ethyl ethanoate (vinyl acetate) or methyl propenoate (methyl acrylate). These fibres, known as acrylic fibres, are discussed in more detail below.
To make plastics.
These are again co-polymers. The two most important are SAN, (styrene-acrylonitrile) a co-polymer of propenonitrile (acrylonitrile) and phenylethene (styrene) and ABS ,(acrylonitrile butadiene styrene) a co-polymer of propenonitrile, butadiene and phenylethene (styrene). These widely-used plastics, which are very resistant to shock, are also discussed further below.
To make propenamide (acrylamide)
The manufacture of propenamide from propenonitrile has provided chemists with a very difficult problem. At first sight, it looks easy, simply the hydrolysis of propenonitrile:
|
|
It is possible, using a copper catalyst at ca 373 K.
Hovever there are disadvantages. The catalyst is difficult to regenerate and unwanted polymerization and conversion to propenoic (acrylic) acid may occur.
A small Japanese company, Nitto, has developed a remarkable method in which the hydrolysis takes place using a microbiological technique. A hydrolyzing enzyme, nitrile hydratase, is immobilised in a gel and at a temperature of 283 K, a pure aqueous solution (up to 20%) of propenamide (acrylamide) is produced.
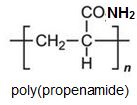
Much of the propenamide (acrylamide) is polymerised to form poly(propenamide) (polyacrylamide) which is widely used as a water-soluble thickening agent (as its name suggests, it increases the viscosity of the solution) and it is found to be very useful in treating waste water and in paper-making where it strengthens the fibres.
Propenamide (acrylamide) is also used as a starting material for the manufacture of diaminohexane, (hexamethylenediamine) and is one of the principal methods of making the diamine, used in the manufacture of polyamide 6,6. The process is known as hydrodimerization.. This term, as it indicates the dimerization of propenamide, with 3 carbon atoms in the molecule, to a species with 6 carbon atoms, together with reduction. This remarkable reaction is carried out electrochemically and the overall reaction can be expressed as:
|
|
The resulting dinitrile is hydrogenated by passing its vapour and hydrogen over a nickel catalyst at ca 500 K, under pressure (ca 35 atm):
|
|
There are a variety of electrolytes used, the first being a tetraalkylammonium salt. This is the only example of the use of electrochemistry to manufacture an organic chemical on a large scale.
To make NBR (Nitrile Butadiene Rubber)
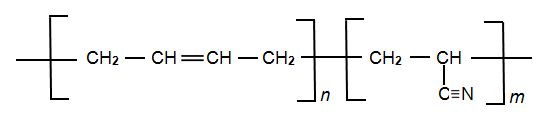
Another synthetic co-polymer made from propenonitrile is NBR (Nitrile Butadiene Rubber), the other monomer being buta-1,3-diene. It is a block co-polymer:
 |
NBR's ability to withstand a range of temperatures from 220 to 380 K and its resistance to attack by oil and many chemicals allows it be used in several important aeronautical applications, for example to make fuel and oil handling hoses and seals, where ordinary rubbers cannot be used. It is also used to make protective gloves for use in the nuclear industry. NBR is also used in everyday items, for example in footwear.
Its physical and chemical properties vary depending on the polymer’s composition, the higher the proportion of nitrile groups (the larger the proportion of m to n (above), the higher the resistance to oils but the lower the flexibility.
The manufacture of propenamide has provided chemists with a very difficult problem. At first sight, it looks easy, the hydrolysis of propenonitrile:

There are disadvantages. The catalyst is difficult to regenerate and unwanted polymerization and conversion to propenoic (acrylic) acid may occur.
A small Japanese company, Nitto, has developed a remarkable method in which the hydrolysis takes place using a microbiological technique. A hydrolyzing enzyme, nitrile hydratase, is immobilised in a gel and at a temperature of 283 K, a pure aqueous solution (up to 20%) of propenamide (acrylamide) is produced.
Uses of poly(propenonitrile) (polyacrylonitrile)
Poly(propenonitrile) itself is a very harsh fibre, rather like horse hair. An almost pure homopolymer is used when a very tough fabric is needed, for example for awnings, a soft top of a car or in brake linings. It is even used to reinforce concrete and in road construction. However, the vast majority of the polymer is co-polymerized. Although these co-polymers often contain more than 85% of propenonitrile units, they are much softer. The fibres formed from them are known as 'acrylic' fibres.
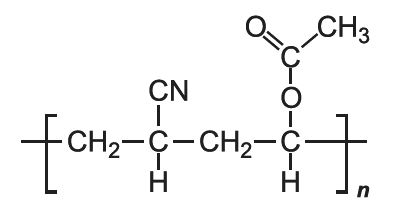
Two of the most used acrylic fibres are formed from the co-polymerization of propenonitrile with ethenyl ethanoate (vinyl acetate) and propenonitrile with methyl propenoate (methyl acrylate). The former is often mixed with cotton fibres to produce a light fabric, used in women's clothes. The latter is often used with wool (Figure 1).
|
The co-polymers with phenylethene (styrene) known as SAN and with butadiene and phenylethene, known as ABS, are plastics which are very strong and able to withstand shocks.
|
Figure 3 The high heels are made from a blend of ABS and a polyamide which is very strong. |
|
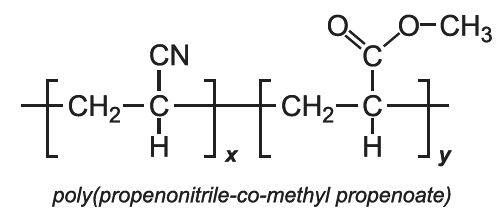
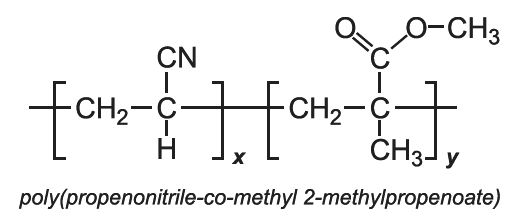
Other co-polymers of propenonitrile include those when the co-monomer is methyl 2-methylpropenoate (methyl methacrylate) and with 1,1-dichloroethene.
With 1,1-dichloroethene as the co-monomer, a block co-polymer is formed which is fire-resistant and is often used in children's clothing.
An increasing use of poly(propenonitrile) co-polymers is in producing carbon fibres. If fibres of the polymer are heated under strictly controlled conditions the resulting fibres have remarkable strength.
Annual production of propenonitrile (acrylonitrile)
| World | 6.9 million tonnes2 |
| Europe | 1.8 million tonnes |
| US | 1.2 million tonnes |
2. Estimate for 2017, Merchant and Consulting Report, published Market Publishers Ltd 2018
Manufacture of poly(propenonitrile) (polyacrylonitrile)
(a) The monomer
Propenonitrile (acrylonitrile), the monomer, is manufactured from propene. The alkene is mixed with ammonia and oxygen (from air) (1:1:2 volume ratio) and passed over a heterogeneous catalyst made from a mixture of bismuth(III) and molybdenum(VI) oxides):
As it is a very exothermic reaction, and the temperature must be controlled at ca 600 K, a fluidized bed reactor is used.
A small amount of hydrogen cyanide (3-6%) is also formed, which can be used in the manufacture of methyl 2-methylpropenoate.
The process has been modified, in Japan, to use propane as the feedstock. It will become particularly important if propane becomes much cheaper than propene. The catalyst used is based on vanadium(V) and antimony(III) oxides.
(b) The polymer
The polymer is manufactured by radical polymerization initiated by either a peroxide or by a mixture of potassium peroxydisulfate, K2S2O8 and a reducing agent such as potassium hydrogensulfite, KHSO3.
About equal amounts of the stereoregular polymer, isotactic and syndiotactic,are produced. The polymerization is either in solution or as a slurry.
Figure 4 The soft tops for high quality cars are produced from almost pure homopolymer.
By kind permission of Valmai Firth.
(c) Co-polymers
Polymerization takes place as for the homopolymer, a radical polymerization. The two monomers are mixed prior to addition of the initiator. When, ethenyl ethanoate is used as the co-polymer, polymerization is initiated with small amounts of potassium hydrogensulfite and potassium peroxodisulfate which get incorporated into the co-polymer, giving it sites which can bind to colorants and make them fast. Alternatively, a small amount of a third monomer containing, for example a sulfonic acid group, serves the same purpose.
The co-polymer contains a more or less regular alternation of the individual monomers, an example of an alternating co-polymer.
Similar procedures are used when other co-monomers are used.
With methyl propenoate and methyl 2-methylpropenoate, block co-polymers are produced:
An alternating polymer is produced with 1,1-dichloroethene as the co-monomer.
Date last amended: 7th January 2019