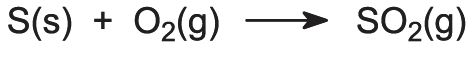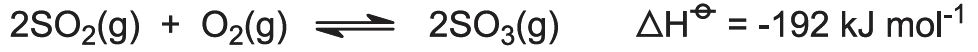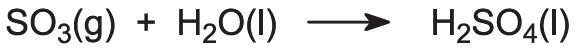Uses of sulfuric acid
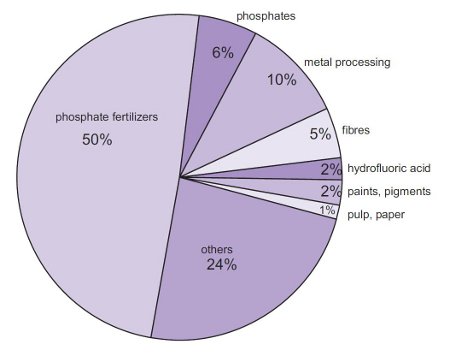
By far the largest amount of sulfuric acid is used to make phosphoric acid, used, in turn, to make the phosphate fertilizers, calcium dihydrogenphosphate and the ammonium phosphates. It is also used to make ammonium sulfate, which is a particularly important fertilizer in sulfur-deficient.
Figure 1 Uses of sulfuric acid.
It is widely used in metal processing for example in the manufacture of copper and the manufacture of zinc and in cleaning the surface of steel sheet, known as 'pickling', prior to it being covered in a thin layer of tin, used to make cans for food.
It is also used to make caprolactam, which is converted into polyamide 6 and in the manufacture of titanium dioxide, used, for example, as a pigment.
Amongst its many other uses is in the manufacture of hydrofluoric acid and phenol with propanone all of which are used in many industries.
Annual production of sulfuric acid
| World | 231 million tonnes |
| China | 74 million tonnes |
| U.S. | 37 million tonnes |
| India | 16 million tonnes |
| Russia | 14 million tonnes |
| Morocco | 7 million tonnes |
Data estimated from:
Merchant Research & Consulting Ltd.
These figures relate to 2011 - 2012. It was expected that by 2012, the World production would be over 250 million tonnes (mcgroup.com) and 260 million tonnes by 2018 (marketsandmarkets.com) with the upward trend forecasted to at least 2023 (transparencymarketresearch.com).
Manufacture of sulfuric acid
The process for producing sulfuric acid has four stages:
a) extraction of sulfur
b) conversion of sulfur to sulfur dioxide
c) conversion of sulfur dioxide to sulfur trioxide
d) conversion of sulfur trioxide to sulfuric acid
(a) Extraction of sulfur
Easily the most important source of sulfur is its recovery from natural gas and oil. These contain sulfur compounds, both organic and hydrogen sulfide both of which must be removed before they are used as fuels or chemical feedstock.
Another important source of sulfur is as sulfur dioxide from metal refining. Many metal ores occur as sulfides and are roasted to form an oxide and sulfur dioxide, for example, in the manufacture of lead:
Other metals manufactured from their sulfide ores include copper, nickel and zinc.
Worldwide about 35% of the sulfur is obtained as sulfur dioxide from sulfide ore roasting and this is increasing, as plants which traditionally passed the sulfur dioxide to atmosphere are recovering it as sulfuric acid. In particular, China makes most of its sulfuric acid from pyrites, an iron sulfide ore.
Sulfuric acid is also obtained from ammonium sulfate, a by-product in the manufacture of poly(methyl 2-methylpropenoate) and also recovered from 'spent' (i.e. used) sulfuric acid.
(b) Conversion of sulfur to sulfur dioxide
If sulfur is the feedstock, it must first be converted to sulfur dioxide. Molten sulfur is sprayed into a furnace and burnt in a blast of dry air at about 1300 K. The sulfur burns with a characteristic blue flame:
As excess air is used the emerging gas contains about 10-12% sulfur dioxide and 10% oxygen, by volume. The gases are very hot and so are passed through heat exchangers (waste heat boilers).
The gases are cooled to about 700 K and the water in the surrounding boiler pipes is converted into steam. In manufacturing one tonne of sulfuric acid, one tonne of high pressure steam is also produced.
(c) Conversion of sulfur dioxide to sulfur trioxide (The Contact Process)
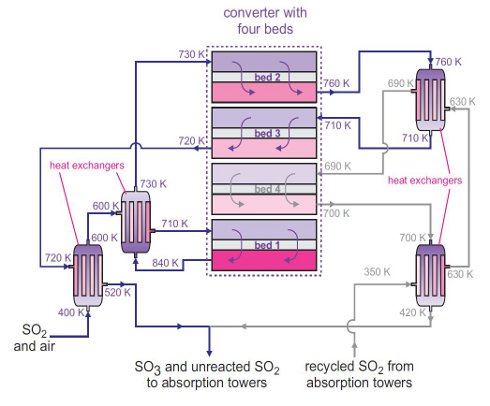
A typical plant contains one cylindrical vessel which acts as a fixed bed reactor with four separate beds of catalyst, known as a converter, heated to 700 K, through which the sulfur dioxide and air pass:
The catalyst, vanadium(V) oxide on silica, is generally in the form of small pellets, to which caesium sulfate has been added as a promoter (Figure 2). The function of the promoter is to lower the melting point of vanadium(V) oxide so that it is molten at 700 K.
| Figure 2 Vanadium(v) oxide catalyst used for the manufacture of sulfuric acid. The gas inlet duct can be seen in the middle of the picture. By kind permission of Haldor Topsøe. |
|
Figure 3 A flow diagram of the Contact Process. As shown above, it is an exothermic reaction so, for a satisfactory yield of sulfur trioxide (above 99.5% conversion is needed), as low a temperature as economically possible is used. Thus, heat is removed from the gas leaving each bed using heat exchangers. The sulfur trioxide produced is removed between the third and fourth beds and flows to the next stage, the conversion of sulfur trioxide to sulfuric acid. |
  |
(d) Conversion of sulfur trioxide to sulfuric acid
The sulfur trioxide formed from the third bed (and the small amount from the fourth bed) are now converted to
sulfuric acid.
Sulfur trioxide reacts with water and the reaction can be expressed as:
However, water itself cannot be used for absorption as there is a large temperature rise, and a sulfuric acid mist is formed, which is difficult to handle. Instead, sulfuric acid of about 98% concentration is used. This is kept at this concentration by addition of water and removal of acid at that concentration.
To keep the temperature at about 400 K, the heat is removed by heat exchangers, Figure 4.
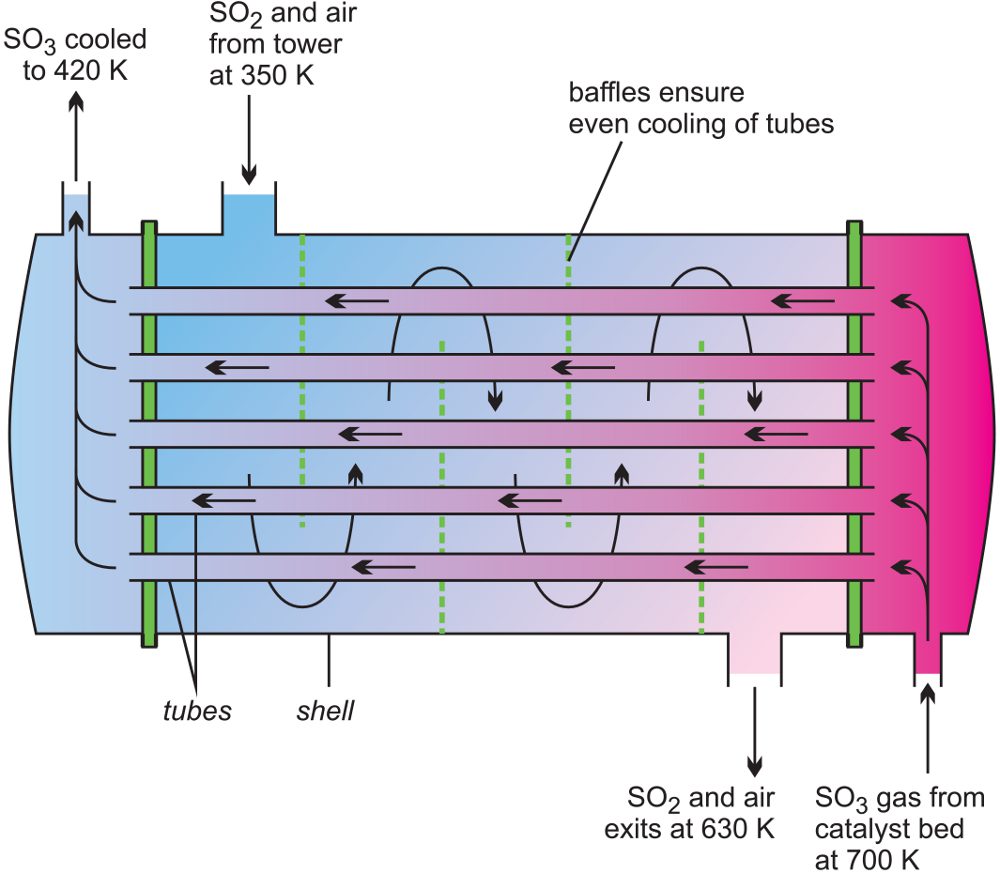
Figure 4 A line diagram illustrating a heat exchanger used in the manufacture of sulfur trioxide.
The gases not absorbed contain about 95% nitrogen, 5% oxygen, and traces of sulfur dioxide. The gas stream is filtered to remove any traces of sulfuric acid mist and is returned to the atmosphere using a high stack.
Date last amended: 9th October 2016