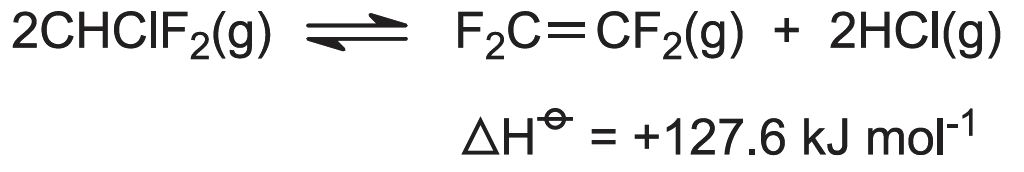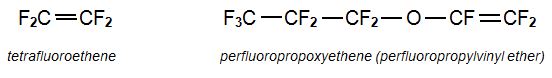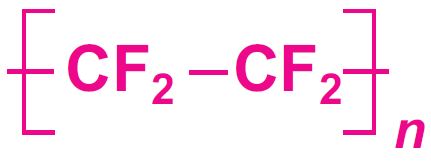
Among the homopolymers, poly(tetraflororethene (PTFE) is the best known fluoropolymer, accounting for about 60% (by mass) of the total amount of the fluoropolymers produced. The other homopolymers include:
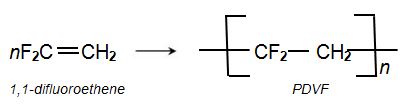
PVDF (poly(1,1-difluoroethene) (polyvinylidene difluoride) and
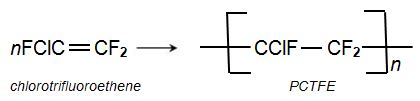
PCTFE (polychlorotrifluororethene).
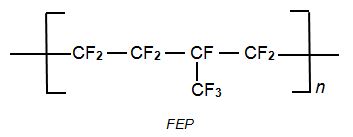
The co-polymers include FEP ,Fluorinated Ethene Propene (fluorinated ethylene propylene),
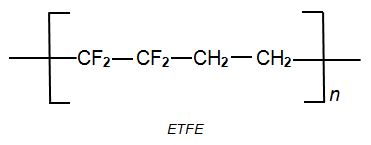
ETFE (a co-polymer of ethene and tetrafluorethene)
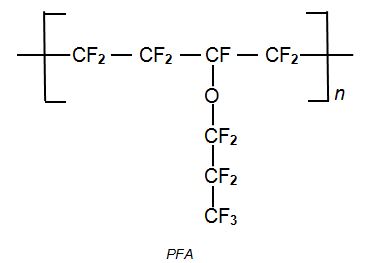
and PFA (a group of perfluoroalkoxy alkane co-polymers)
The uses of the fluoropolymers are a function of their properties such as resistance to chemical attack, thermal stability, low friction and non-stick properties and high electrical resistance (Table 1). Their properties vary with their structure and so there is a range of fluoropolymers from which to choose for a particular purpose, although cost must also be a factor.
| Property | Use |
|---|---|
| High thermal stability | Can be used between 60 to 630 K |
| High melting point | From 453 K (PVDF) to 600 K (PTFE) |
| Stable to chemical attack | Can be used in contact with mineral acids, alkalis, inorganic oxidizing agents, solvents (for example, alcohols, ketones, esters, chlorocarbons and fluorocarbons) |
| Low dielectric constant | Excellent insulator |
| Low coefficient of friction | Little friction between moving parts |
| The cohesive forces between polymer and water are much less than between water molecules | Imparts non-stick properties to utensils |
| Stable to UV radiation | Can be used outside in sunlight |
Table 1 Some properties of fluoropolymers
The fluoropolymers can also be reinforced. The filler for PTFE, for example. is usually glass fibres, with the percentage varying between 5% and 40% depending on the needs of the application. As the filler proportion increases so do the properties that the filler offers (for example increased compressive strength means lower deformation under load). However, the coefficient of friction properties of the material also increases over that of virgin PTFE and this may not be desirable for some purposes.
Uses of poly(tetrafluoroethene) (polytetrafluoroethylene) and other fluoropolymers
Poly(tetrafluoroethene) (PTFE) and other fluorinated polymers are used in:
• cable insulation for electronics including aerospace
• reactor and plant equipment linings, when reactants or products are highly corrosive to ordinary materials such as steel
• semi-permeable membranes in chlor-alkali cells and fuel cells
• bearings and components in mechanical devices such as small electrical motors and pumps
• permeable membrane (e.g. Gore-TexTM), for clothing and shoes, which allows water vapour to diffuse away from the skin but prevents liquid water (rain) from soaking in
• coating of clothing used in medicine (gowns, sheets)
• non-stick domestic utensils, e.g. frying pans
• medical - catheter tubing
• hose and tubing
• solid lubricants
• combinations with magnesium and aluminium as an igniter for explosives
• fibres for clothing
It is likely to be the automotive and transportation markets that provide much of the growth in the use of PTFE in the next few years. One such is ion lithium-ion batteries which are used in many advanced devices. Small ones include mobile phones and tablets and pacemakers. They are also used in electric cars and in large satellites and interplanetary explorers.
PVDF and FEP are used as binders for both the cathode (for example graphite) and anode (for example lithium cobalt oxide).
|
|
| Figure 1 The retractable roof of the Centre Court at Wimbledon is made of poly(tetrafluoroethene). In this photo, the roof is being closed. By kind permission of AGC Chemicals Europe Ltd. |
Annual production of poly(tetrafluoroethene) (polytetrafluoroethylene) and other fluoropolymers
PTFE
| World | 200 000 tonnes1, 2, 3 |
| Europe | 15 000 tonnes4 |
PDVF
| World | 46 100 tonnes5 |
FEP
| World | 28 400 tonnes6 |
ETFE
| World | 19 130 tonnes7 |
1. In 2015, Research and Markets, 2016
2. Expected to be 247 000 tonnes by 2022, Research and Markets, 2016
3. China accounted for over 50% of the global production in 2015. HIS Markit 2016
4. The Statistics Portal, Statista 2018
5. Expected to be 69 000 tonnes by 2022, Industry Experts, 2016
6. Expected to be 41 100 tonnes by 2022, Research and Markets, 2016
7. Projected for 2019. The tonnage produced in 2014 was 12 950, The Statistics Portal, Statista, 2018
Manufacture of poly(tetrafluoroethene) (polytetrafluoroethylene) and other fluoropolymers
PTFE is made from methane in a series of reactions:
a) production of trichloromethane (chloroform)
b) production of chlorodifluoromethane
c) production of tetrafluoroethene (TFE)
d) polymerization of tetrafluoroethene
(a) Production of trichloromethane (chloroform)
Trichloromethane is one of the products formed by the reaction of methane and a mixture of chlorine and hydrogen chloride. This can be performed in the liquid phase at 370-420 K using a zinc chloride catalyst. Alternatively, the reaction is carried out in the vapour phase, using alumina gel or zinc oxide on silica as a catalyst at 620-720 K.
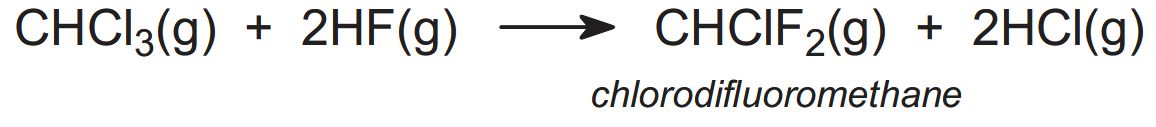
(b) Production of chlorodifluoromethane
Trichloromethane is reacted with anhydrous hydrogen fluoride in the presence of antimony(III) and antimony(V) chlorofluoride to give chlorodifluoromethane:
(c) Production of tetrafluoroethene (tetrafluoroethylene, TFE))
Since TFE is an explosive gas (bp 197 K), it is usually made when and where required for polymerization so that there is minimum storage time of the monomer between its production and its polymerization.
Chlorodifluoromethane is heated in the absence of air, a process known as pyrolysis:
Low pressures (atmospheric) and high temperatures (940-1070 K) favour the reaction.
Steam, preheated to 1220 K, and chlorodifluoromethane, at 670 K, are fed into a reactor. Steam is used to dilute the reaction mixture and hence reduce the reactant partial pressure, and thus the formation of carbon and toxic by-products. The steam also supplies all the heat required by this endothermic reaction. Very little hydrolysis of reactant and product occurs.
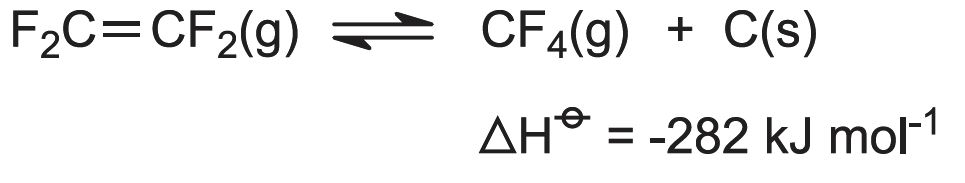
Once formed, the product must be rapidly cooled to 770 K to prevent the reverse reaction occurring and the explosive decomposition of TFE:
The cooling is done by passing the vapour through a water-cooled heat exchanger, made of graphite to resist chemical attack and thermal shock. Reactor residence time is 1 second.
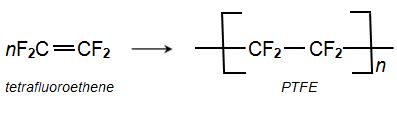
(d) Polymerization of tetrafluoroethene (tetrafluoroethylene)
The monomer is transformed into the polymer poly(tetrafluoroethene) (PTFE), by radical polymerization. The reaction is carried out by passing TFE into water containing a radical initiator, e.g. ammonium persulfate, (NH4)2S2O8, at 310-350 K and a pressure of 10-20 atm.
Two different procedures are used:
• suspension polymerization (sometimes known as granular polymerization) gives a suspension of string-like PTFE particles (with a high relative molecular mass from 5 to 500 million) up to 1 cm long in water. These are milled to produce fine powders (30 µm) used for moulding. The fine powders are also agglomerated to larger particles (50-500 µm) to give better flow. Unlike other thermoplastics, such as PVC, PTFE cannot be processed by melt extrusion. The powder is therefore moulded into rods for extrusion and heating at temperatures above 530 K to force the particles to stick together.
About a third of the PTFE is produced in this form. Fillers are often added prior to the production of sheets and rods.
• dispersion polymerization is used to obtain a colloidal dispersion of PTFE particles (0.1-0.3 µm) in water. The dispersion can be concentrated and used for dip coating or spraying articles. The dispersion can also be coagulated and dried to give a fine powder, which, in turn, is made into a paste and extruded on to wire. The wire is used extensively in cars and aerospace when it is subjected to high temperatures.
The polymer has a relative molecular mass from 1 to 5 million
About half the PTFE is produced in this form.
About 20% of the PTFE formed from either of the two processes above and left over from machining is converted by degradation to a micropowder which is used in paints, inks and lubricants. The powder is fine,1 µm or below. This degradation is achieved using an electron beam, cobalt irradiation or heat.
Other fluoropolymers
The other homopolymers and co-polymers containing fluorine are, like PTFE, made by free-radical polymerization. The process is usually carried out in water at low temperatures (273-373 K) with a water-soluble peroxide, such as ammonium persulfate, as catalyst.
The most important homopolymer, after PTFE is PVDF.
Poly(1,1-difluoroethene) or poly(vinylidene difluoride (PVDF) is produced using 1,1-difluorethene (vinylidene difluoride) as the monomer in place of tetrafluorethene used when producing PTFE.
A third homopolymer is poly(chlorotrfluoroethene) (PCTFE) for which the monomer is chlorotrifluoroethene:
There is a group of co-polymers which are formed by the co-polymerization of tetrafluoroethene and other unsaturated organic compounds such as ethene, hexafluoropropene and perfluoropropylvinyl ether. They are all alternating co-polymers.
FEP, Fluorinated Ethene Propene (Fluorinated Ethylene Propylene) is formed from a mixture of tetrafluoroethene and perfluoropropene (hexafluoropropene), it is an alternating co-polymer.
ETFE is a co-polymer of tetrafluoroethene and ethene:and is usually known by its trivial name, ethylene tetrafluoroethylene (ETFE):
It is used, in particular as a lining for containers as it is stable to attack by concentrated solutions of acids and alkalis, and because of its good electrical properties of insulation and its strength, it is used as a coating for wires and cables. Its most spectacular use is as a roofing material in buildings such as the O2 Arena in London, the Eden Project in Cornwall, England and the Birds Nest Olympic Stadium in Beijing. The roofs are made up of 2 - 5 layers of large cushions of ETFE. It is also used as an outer skin of large buildings (Figure 2).
| Figure 2 The outer skin of the Allianz Arena in Munich is made of cushions of ETFE. There are lights inside the cushions which are changed depending on which team is playing: white when the German national team is playing, red for FC Bayern Munich and blue for TSV1860 Munich. By kind permission of AGC Chemicals Europe. |
 |
PFA, perfluoroalkoxyalkanes are another group of co-polymers. One example is formed from tetrafluoroethene and perfluoropropoxyethene (perfluoropropylvinyl ether).:
PFA polymers have similar properties to those of PTFE but unlike PTFE they can be melt processed.
Date last amended: 28th November 2018