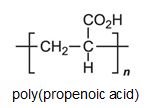
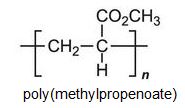
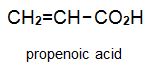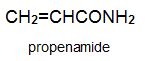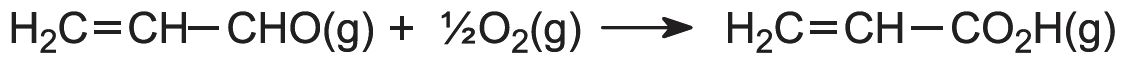There is a group of polymers, the acrylics, which can be regarded as based on acrylic acid, more formally named propenoic acid.
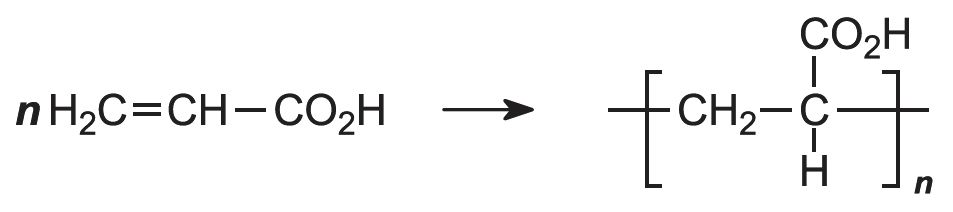
The acid polymerizes by addition polymerization to poly(propenoic acid) (polyacrylic acid).
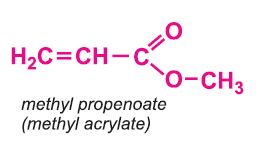
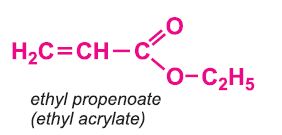
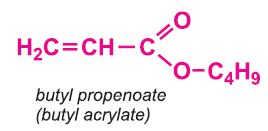
Other compounds, based on the acid, which form acrylics include the methyl, ethyl and butyl esters of propenoic acid.
 |
 |
 |
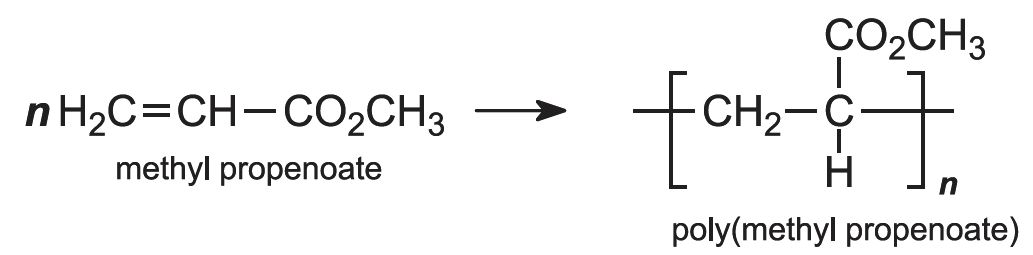
Each of these esters can be polymerized, for example the methyl ester forms this acrylic:
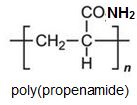
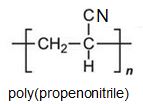
Two other important monomers, based on the acid, are propenonitrile (acrylonitrile) and propenamide (acrylamide).
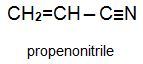 |
|
It can be seen that the polymers have the basic structure of the acrylics shown above.
 |
|
Poly(methyl 2-methylpropenoate, (poly(methyl methacrylate)), is another important polymer and is known simply as acrylic or acrylic glass. It is based on the methyl ester 2-methylpropeonic acid (methacrylic acid).
This website contains three units on the acrylics:
Poly(propenoic acid) (Polyacrylic acid)
Poly(propenonitrile) (Polyacrylonitrile)
Poly(methyl 2-methylpropenoate) (Poly(methyl methacrylate))
This unit is concerned with propenoic acid (acrylic acid), its esters and the polymers produced from them.
Uses of poly(propenoic acid) (polyacrylic acid)
Poly(propenoic acid) is used in detergents to remove calcium and magnesium ions from the water, thus softening it. This has meant that phosphates need not be used for this purpose thus producing a much more environmentally friendly detergent.
|
A second use is the production of the so-called superabsorbents. These are polymers of mainly propenoic acid and sodium propenoate. Polymerization is initiated with, for example, potassium (or ammonium) peoxodisulfate, K2S2O8, which decomposes to form radicals. Another compound is added at the same time to cross-link the chains via the carboxyl groups. One of these compounds is N,N'-methylenebis(2-propenamide). A gel is formed which absorbs water more than 1000-fold its mass (Figure 1), and is used as the basis of disposable nappies. About 40% of the acid is used in this way and about 20% in water treatment1.
1. Market Research Store quoted by GlobeNewswire 2018
Uses of polypropenoates (polyacrylates)
The polymers derived from the esters of propenoic acid are used as a base in many paints and varnishes. The polymers of ethyl and butyl propenoates are used in water-based emulsion paints, as is the co-polymer of butyl propenoate and methyl (2-methylpropenoate).
Methyl propenoate is used to produce a co-polymer with propenonitrile which is one of the most widely used 'acrylic' fibres.
Methyl and ethyl propenoates are co-polymerized with methyl 2-methylpropenoate to assist in the fabrication of poly(methyl 2-methylpropenoate), the range of polymers such as Perspex.
Uses of propenoic acid (acrylic acid)
About 50% is used to make esters, mainly methyl, ethyl and butyl propenoates. These are, in turn, polymerized (see below).
About 30% is used to make poly(propenoic acid) and thus superabsorbents.
Annual production of propenoic acid (acrylic acid)
| World | 5.9 million tonnes2 |
2. In 2014 Market Reseach Store quoted by GlobeNewswire 2018
Manufacture of propenoic acid (acrylic acid)
Propenoic acid is manufactured from propene in two steps.
The first stage is the oxidation of propene to propenal (acrolein). The alkene and air are mixed and passed over a heated heterogeneous catalyst, often a mixture of bismuth(III) and molybdenum(VI) oxides on silica, at ca 650 K:
The second stage occurs when propenal and air are passed over another catalyst, a mixture of vanadium(V) and molybdenum(VI) oxides on silica at ca 550 K:
Manufacture of poly(propenoic acid) (polyacrylic acid)
The polymerization of propenoic acid is a free radical process, using an organic peroxide as an initiator. It can be carried out with the pure monomer (known as bulk polymerization), but more often it is polymerized in an aqueous solution or as an emulsion, also in water:
Manufacture of the polypropenoates (polyacrylates)
Propenoic acid is reacted with an alcohol (for example, methanol, ethanol, butan-1-ol) in the liquid phase with a trace of sulfuric acid as a catalyst to produce the esters. For example:
Subsequently the esters are polymerized, by a free radical process, using an organic peroxide as an initiator. The pure monomer may be used (known as bulk polymerization), but again more frequently the reaction is carried out in an aqueous solution or in an emulsion in water. For example:
If co-polymers of the esters are required, the two monomers are mixed prior to the polymerization reaction under similar conditions.
A postscript on propene
All the propenoate (acrylic) polymers are derived from propene as the summary shows:
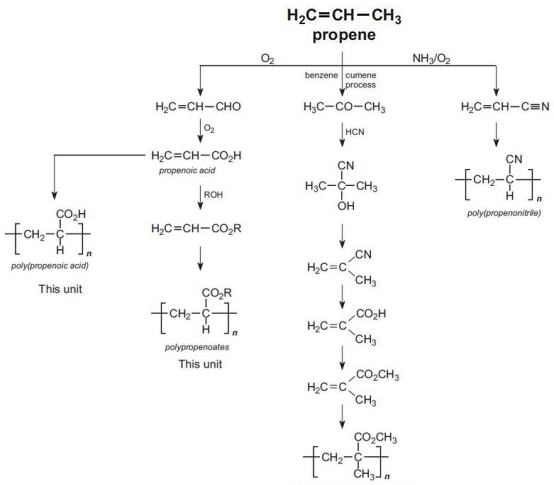
Figure 2 Illustrating the manufacture of poly(propenoic acid), polypropenoates, poly(methyl 2-methylpropenaoate) and poly(propenonitrile) from propene.
Date last amended: 29th October 2018