>Biotechnology is defined as the application of the life sciences to chemical synthesis. This unit discusses its increasingly important role in the direct production of speciality chemicals via fermentation, such as citric acid, lactic acid, propane-1,3-diol and some amino acids.
Other uses of biotechnology are discussed elsewhere, for example the production of biofuels(bioethanol and biodiesel), the production of basic feedstocks such as synthesis gas (carbon monoxide and hydrogen) from biomass and the production of biodegradable polymers such as the poly(hydroxyalkanoates).
The biotechnology industry has a long history in the UK. As long ago as 1916, in the First World War, the bacterium Clostridium acetobutylicum was fed with mashed potato and corn (both containing starch) to produce a mixture of propanone (acetone), butanol and ethanol (known as the ABE process). The propanone was required to produce cordite for munitions. At one time ABE was second only to the production of ethanol as the largest industrial fermentation process. However, the rise of the petrochemical industry in the 1950s provided much cheaper feedstocks for making chemicals, sending the ABE industry into decline around the world.
| Figure 1 Many biochemical reactions in industry are carried out in batch reactors. These reactors are in a bioethanol production unit and are used to treat waste products, carbon dioxide, recycled heat and water to mass-produce algae. Algae from this facility are used for animal feeds, pharmaceutical and beauty products. With kind permission from the U.S. Department of Agriculture/ Lance Cheung (Wikimedia Commons). |
 |
Petrochemicals are a finite resource which will become more expensive as oil becomes scarce, and their use is associated with the release of greenhouse gases that lead to global warming. Producing more chemicals using biotechnology could reduce our dependence on natural gas and oil and reduce the environmental impact of the chemical industry. Some chemicals, such as 2-hydroxypropane-1,2,3-tricarboxylic acid (citric acid), have for many years been routinely produced on the million-tonne scale using biotechnology, as the chemical synthetic routes are complex and expensive. Other notable examples are described in this unit but there are many other processes still in the developmental stage, with chemicals being produced in small reactors on the scale of a few tonnes. A huge amount of research is being done by chemists, biotechnologists and engineers to make these reactions more efficient and cost-effective.
The most important chemicals produced directly by fermentation include 2-hydroxypropane-1,2,3-tricarboxylic acid (citric acid), 2-hydroxypropanoic acid (lactic acid), propane-1,3-diol and amino acids, and each of these is discussed in this unit.
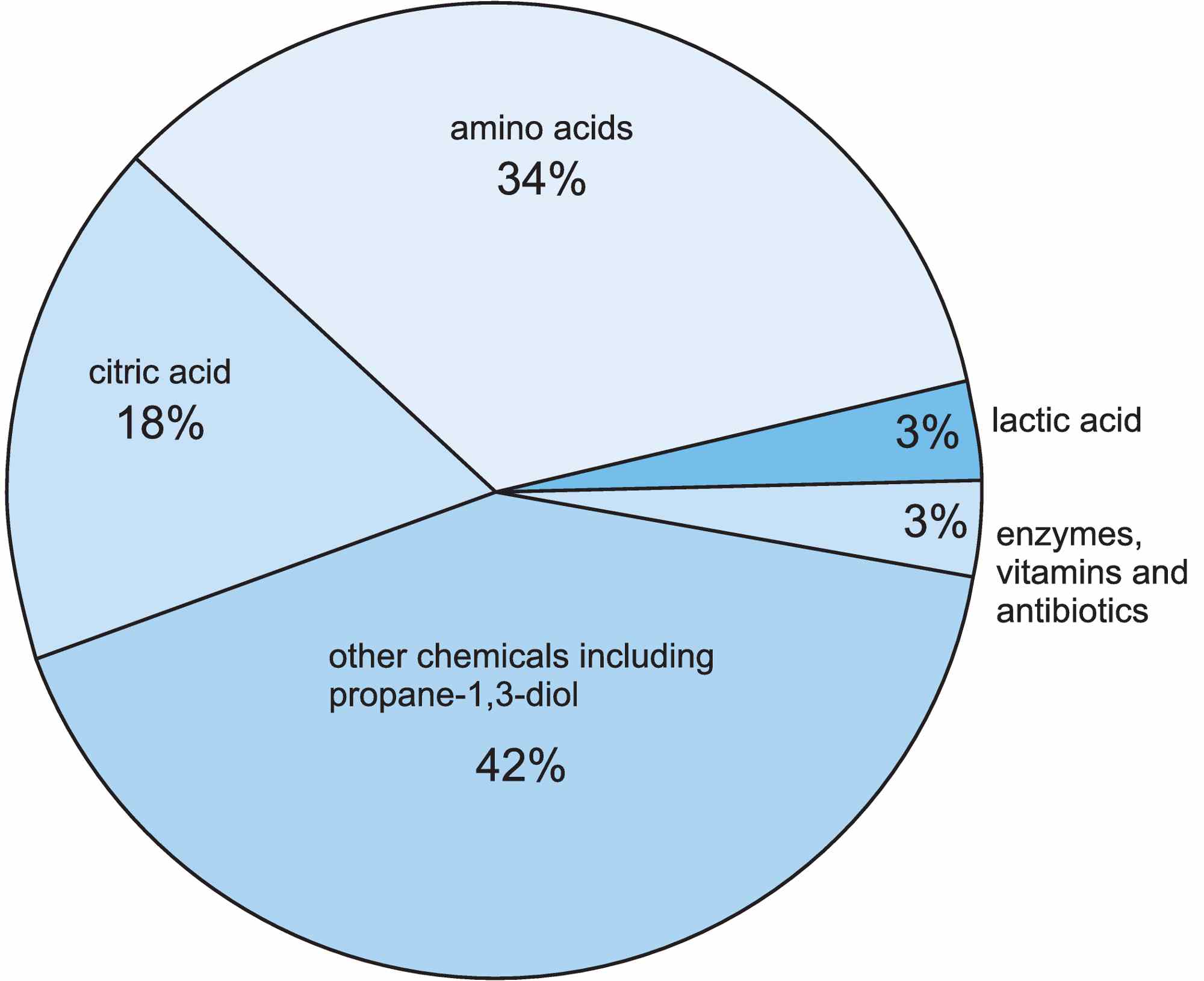
Figure 2 The compounds (other than ethanol) produced by fermentation reactions in the chemical industry.
3-Carboxy-3-hydroxypentanoic acid (citric acid)
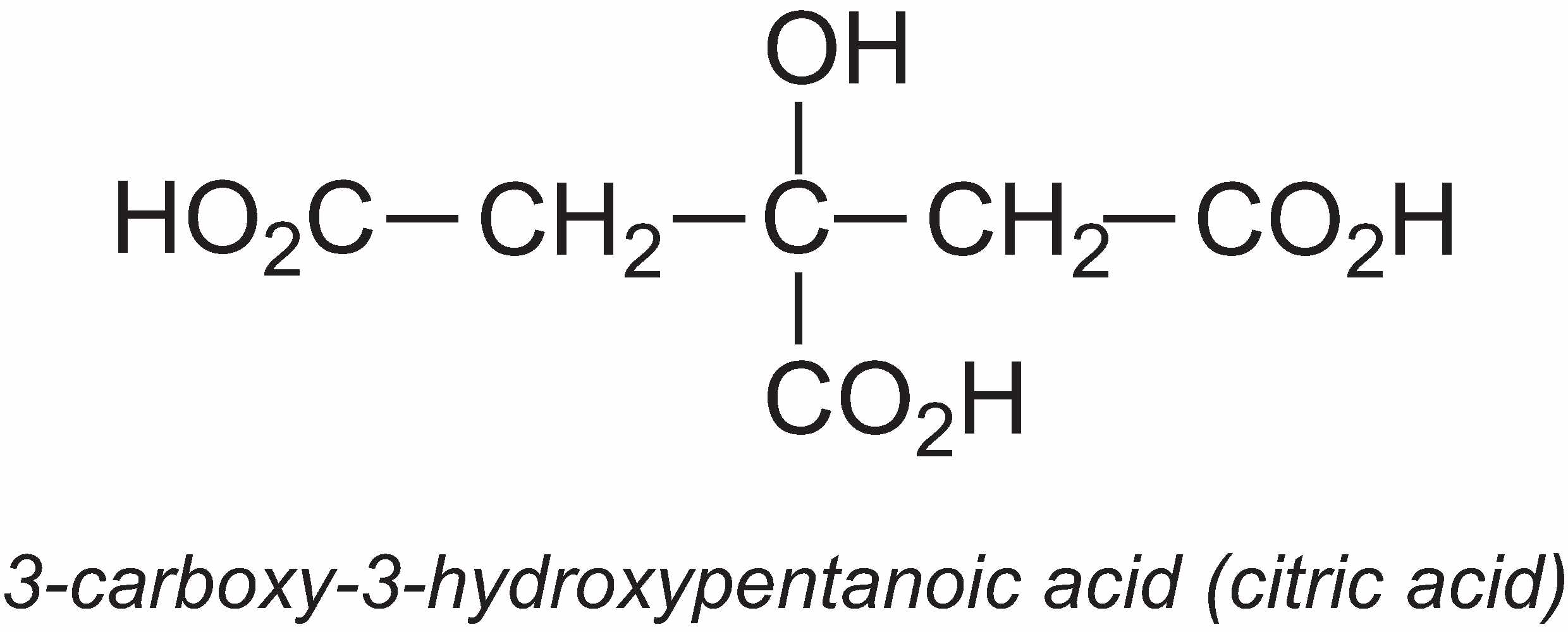
The annual global production of citric acid is about 1.4 million tonnes.
Uses
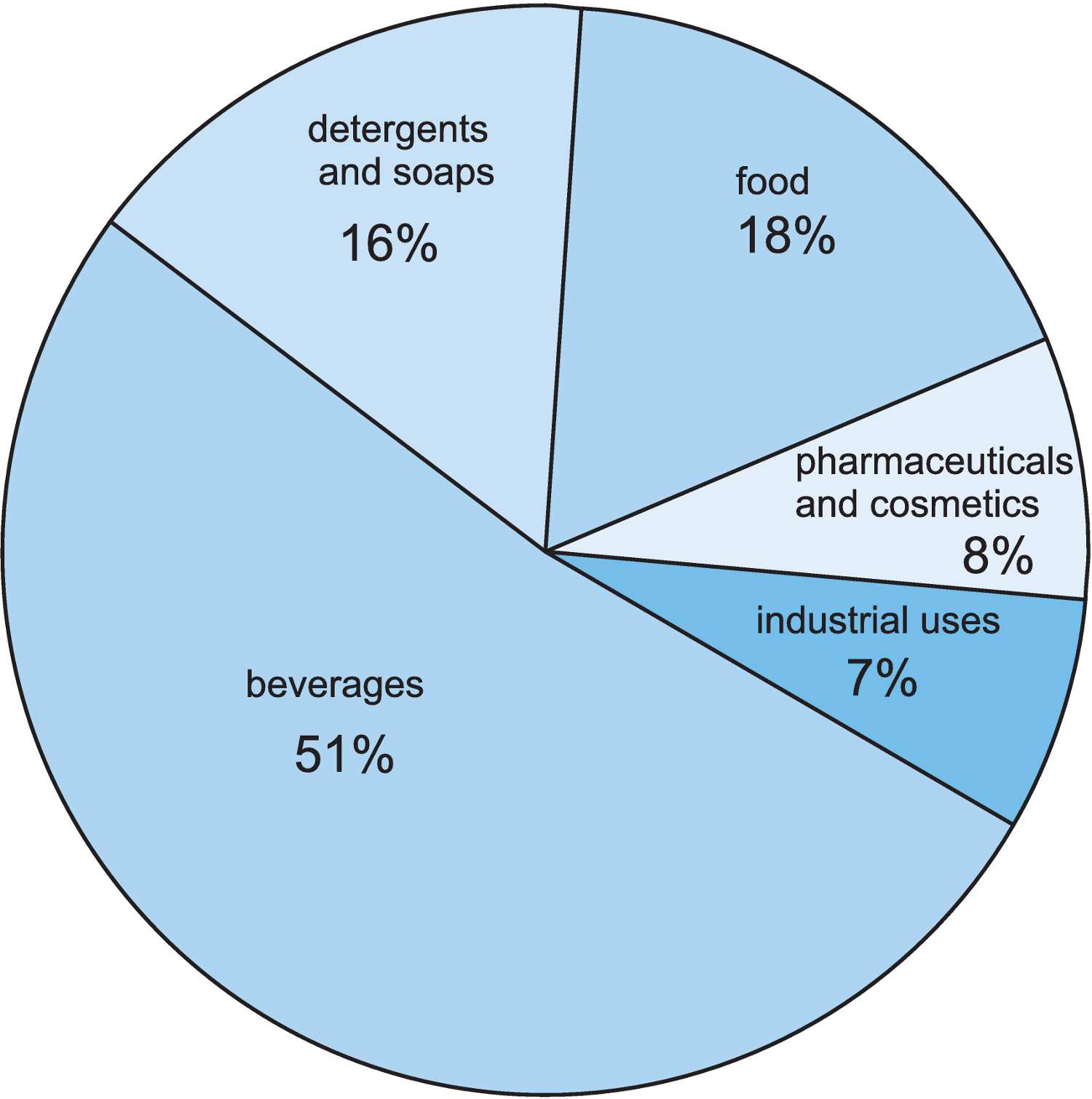
Figure 3 The uses of citric acid.
Citric acid's major use is in the food industry as an acidulant in soft drinks, as a flavouring and as a preservative. It is often listed as the E number E330.
It is used with sodium hydrogencarbonate in effervescent products, both for ingestion (aspirin or antacids) and for personal care (bath salts). It is also used in detergents and soaps to control pH and to chelate metal ions in hard water, which allows detergents to produce more foam (Unit 19).
Manufacture
The main production route uses of the fungus Aspergillus niger, which is grown in solutions of sucrose or glucose. The citric acid produced is precipitated with calcium hydroxide solution to form calcium citrate. This salt is filtered off and the acid regenerated with sulfuric acid.
2-Hydroxypropanoic acid (lactic acid)
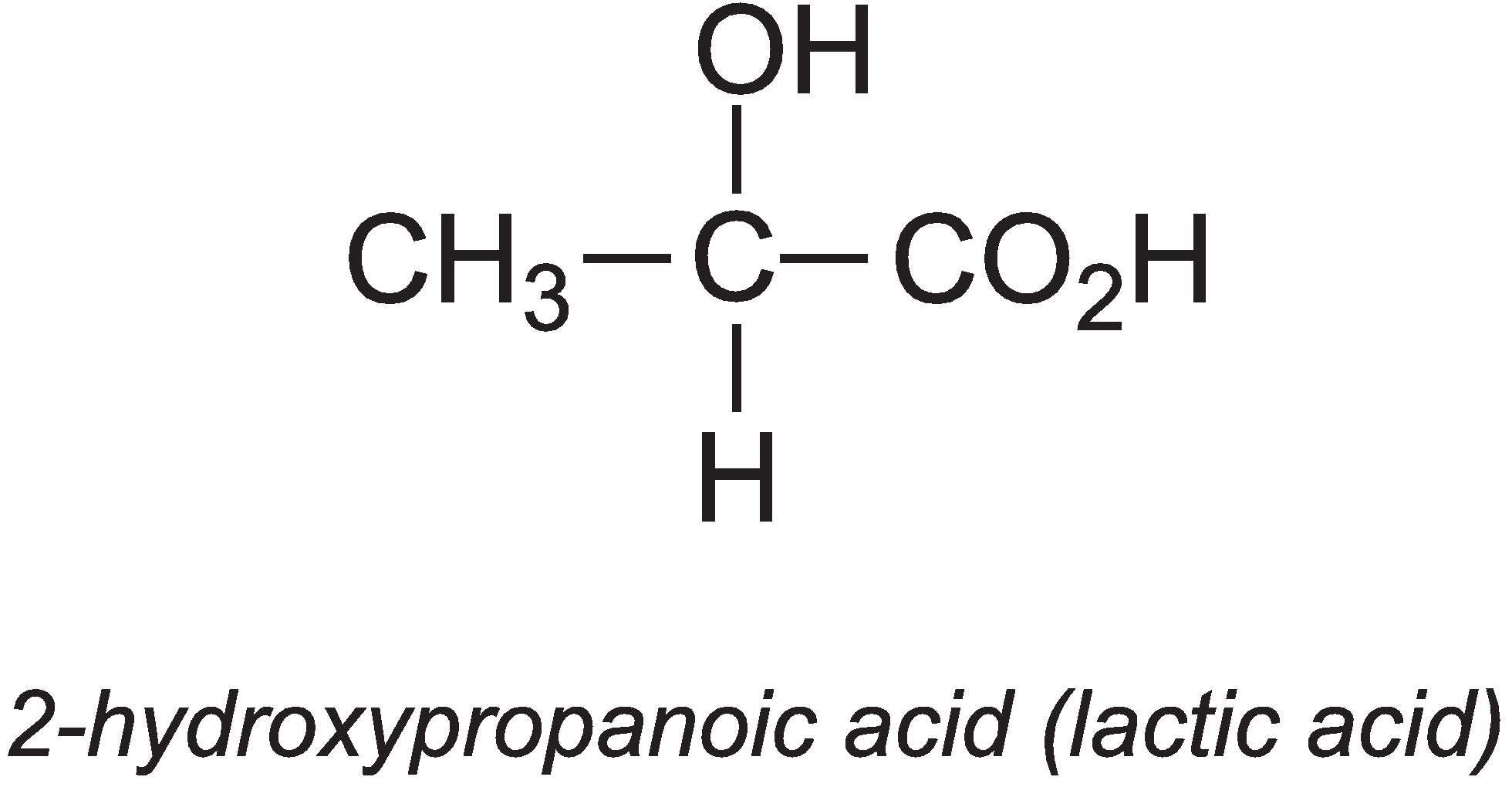
Annually, about 275 000 tonnes of lactic acid are produced globally.
Uses
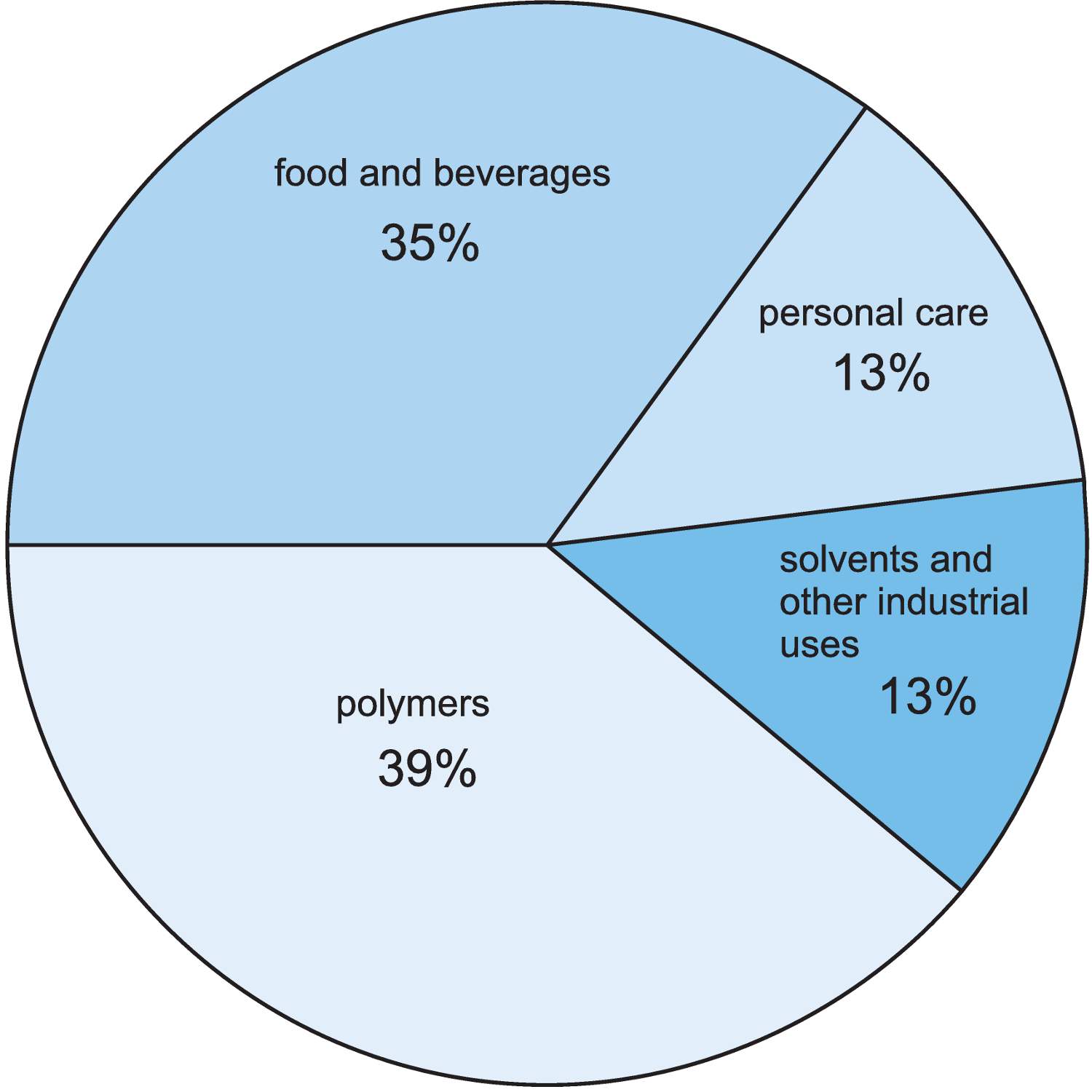
Figure 4 The uses of lactic acid.
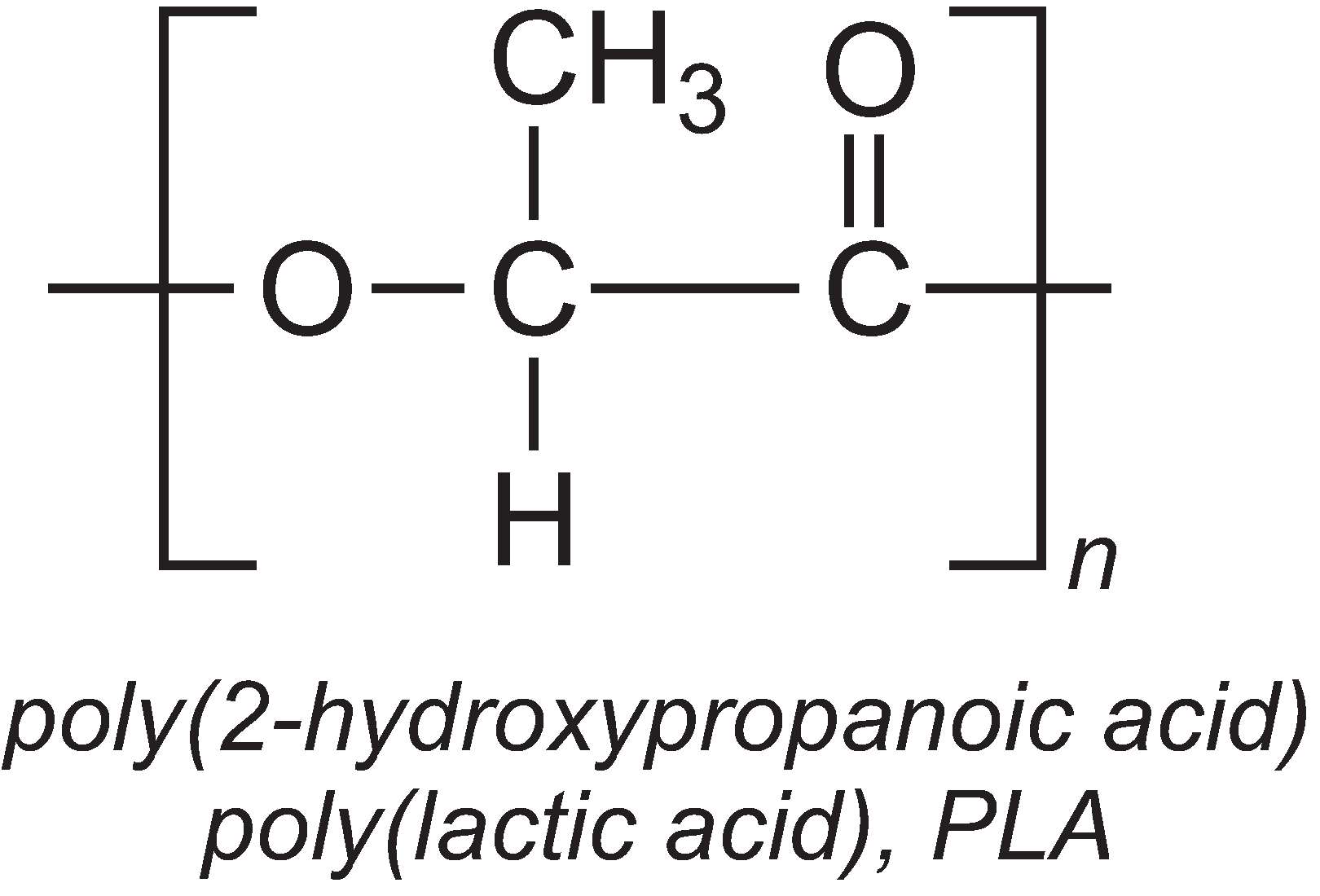
An important use is in the manufacture of the biodegradable polymer, poly(lactic acid), PLA.
Another major use of lactic acid is in food and drinks, as a preservative (it is an anti-oxidant) and to adjust the pH.
Lactic acid can be esterified with ethanol to form ethyl 2-hydroxypropanoate (ethyl lactate) which is a non-toxic and biodegradable solvent. Lactate ester solvents are replacing more toxic substances such as halogenoalkanes as solvents in inks, paints, cleaners and degreasers.
Although lactic acid can be directly polymerized in a condensation reaction, the reaction is reversible and the water that is produced tends to hydrolyze the polymer chain. Instead, lactic acid is first dimerized to make a cyclic lactide. This produces water which is removed.
The lactide is then polymerized into PLA by a ring-opening polymerization reaction, using tin(II) octanoate as catalyst:
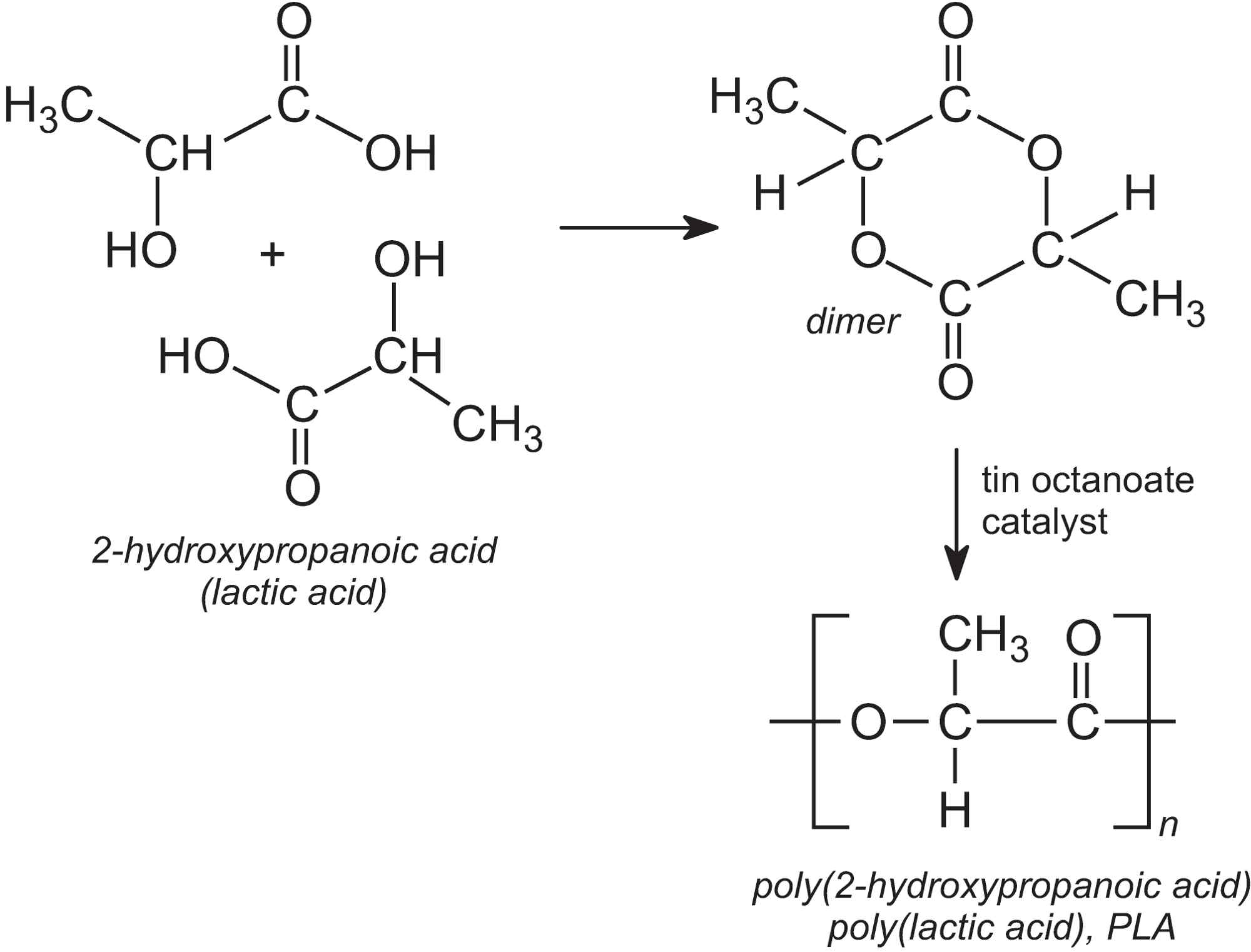
Manufacture
Lactic acid (2-hydroxypropanoic acid) is produced by fermentation of sugars from maize (corn syrup) and cane sugar (molasses) using a lactobacillus bacterium.
Propane-1,3-diol
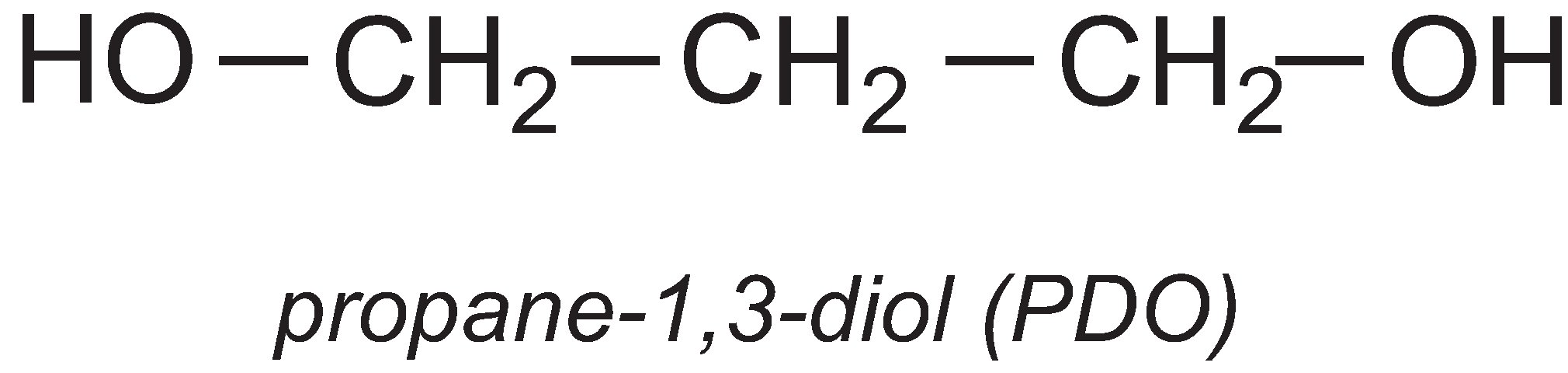
A particularly important use of propane-1,3-diol (PDO) is in the manufacture of the polyester, polytrimethylene terephthalate (PTT). PTT is formed by the condensation reaction between PDO and benzene-1,4-dicarboxylic acid (often called terephthalic acid). The most commonly used catalysts for the reaction are titanium alkoxylates such as tetrabutyl titinate(IV).
PTT is very similar in structure to the well-known polyester polyethylene terephthalate (PET), which is produced from ethane-1,2-diol and benzene-1,4-dicarboxylic acid (Unit 59).
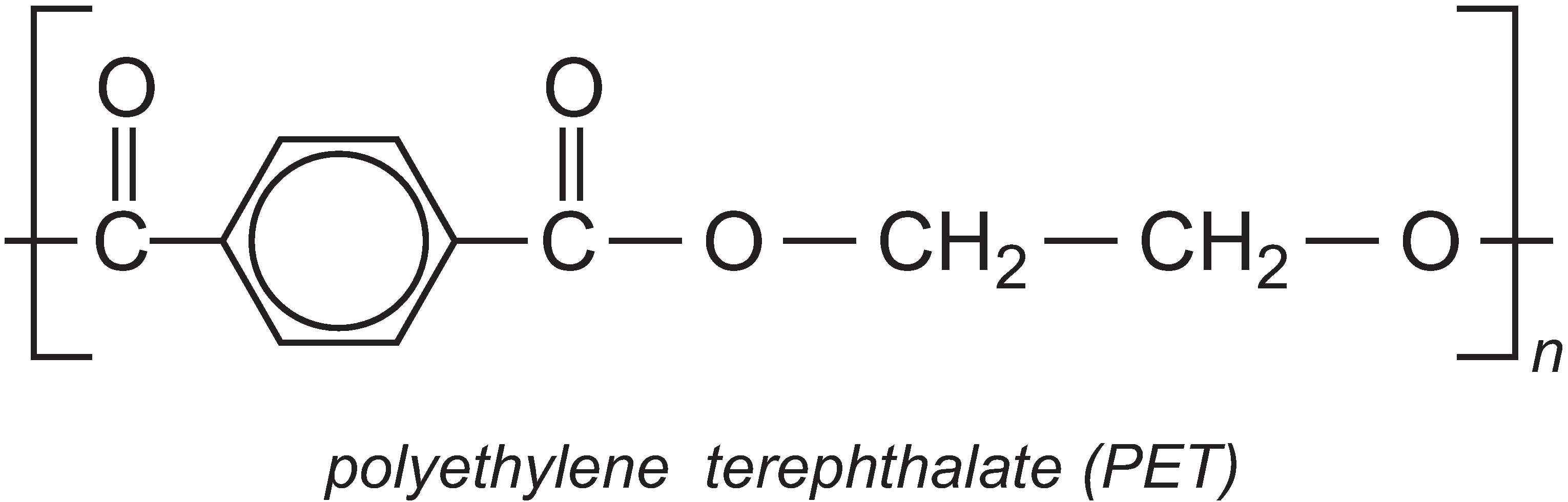
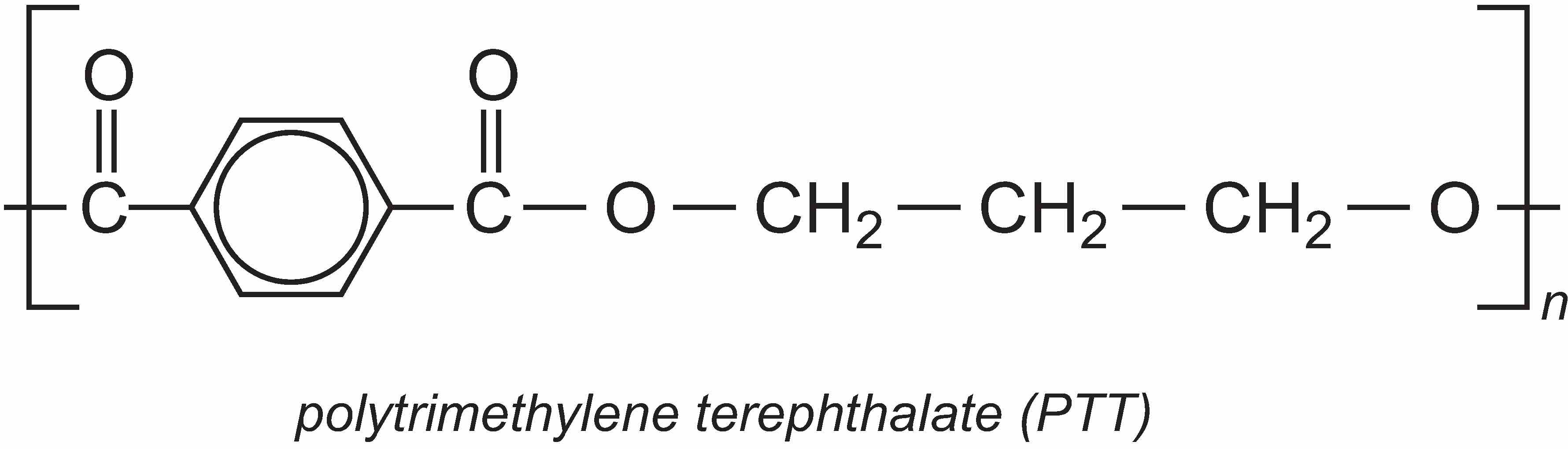
However, the extra methylene groups in PTT gives the polymer pronounced kinks in the chain and hence different properties to PET. PTT is felt to have superior stretch-recovery properties to PET and it is also easy to dye (Unit 11). It is being increasingly used in fibre form in textiles, clothes and carpets, but it can also be used as a thermoplastic in car parts, electrical and electronics systems.
Propane-1,3-diol is also used in the preparation of cosmetics, laminates, adhesives, paints and inks. It is also being used as a replacement for ethane-1,2-diol as an engine coolant and as a solvent.
 |
|
Figure 5 The carpet contains 37% of polytrimethylene terephthalate derived from maize via propane-1,3-diol. |
Manufacture
Propane-1,3-diol is manufactured from maize (corn). The maize is cooked, and then ground to release the starch. The starch is hydrolyzed to produce glucose. This is fed to a genetically modiied Escherichia coli, which ferments the glucose into PDO.
Amino acids
Amino acids contain both amino and carboxyl functional groups. Linear chains of amino acids are the building blocks of proteins. Industrially, amino acids are used in food additives, animal feeds and pharmaceuticals.
With the exception of aminoethanoic acid (glycine), the industrially produced amino acids are chiral and the two isomers (D and L) have different properties in biologically induced reactions. However, chemical synthesis produces equimolar quantities of D- and L- forms and additional expensive steps are required to produce a pure stereoisomer. However, biotechnological routes have the great advantage of producing pure optically active amino acids.
Amino acids are produced from the fermentation of sugar to which small quantities of nitrogen-containing compounds (for example, ammonia or urea) have been added. Mutants of Corynebacterium glutamicum or genetically modified E. coli are used. The two acids produced on the largest scale are L-glutamic acid and L-lysine.
L-Glutamic acid
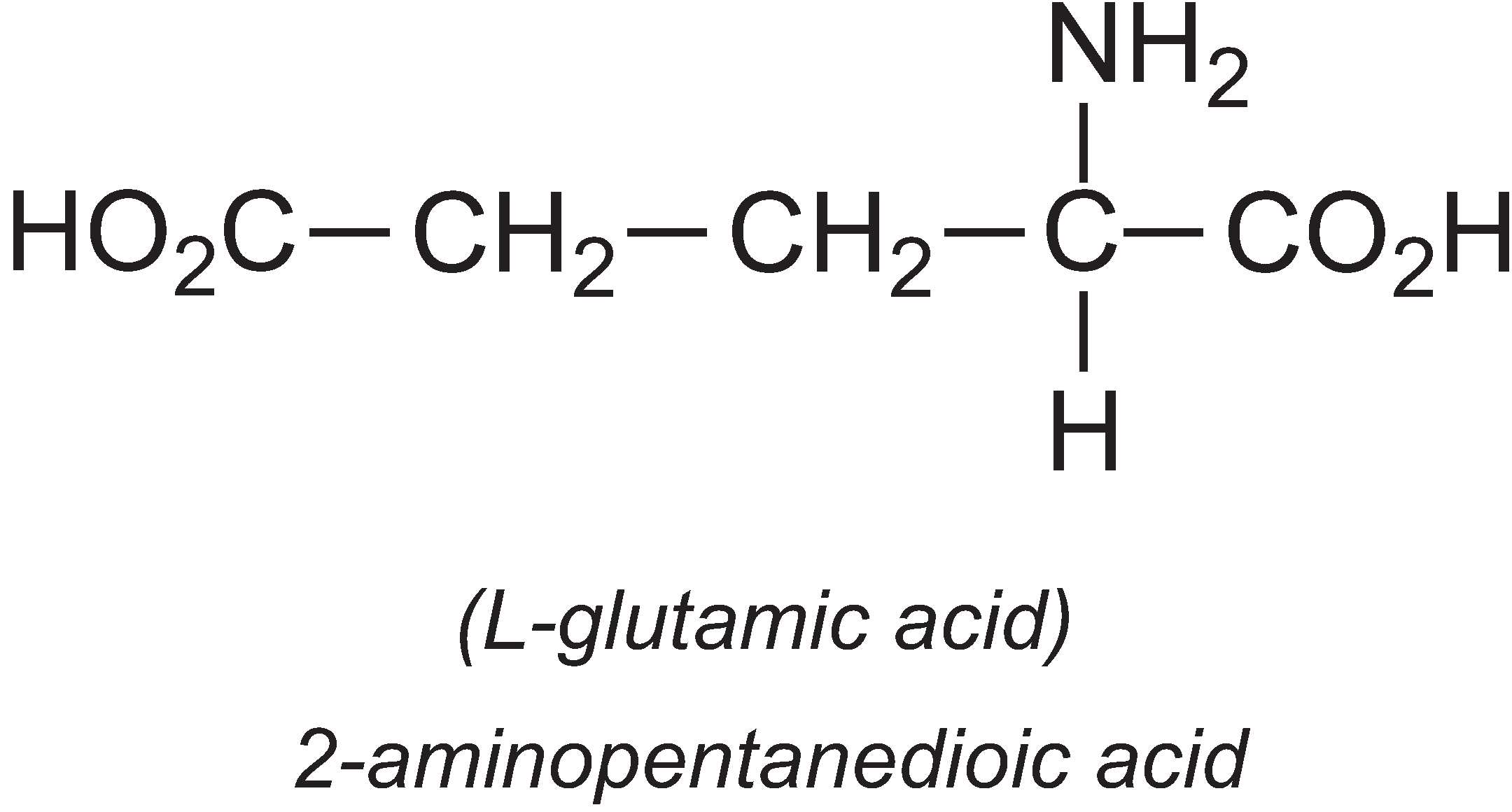
About 1.7 million tonnes of L-glutamic acid are produced by fermentation per year, the majority being produced in Asia, with one company in China producing 33% of the world's output.
Most is used in the form of the salt, monosodium glutamate (MSG) which is commonly used as a flavour enhancer, particularly in processed food. The sodium salt is used rather than the acid as it is the glutamate ion that produces flavour and the salt is more soluble in water than the parent acid.
L-Lysine
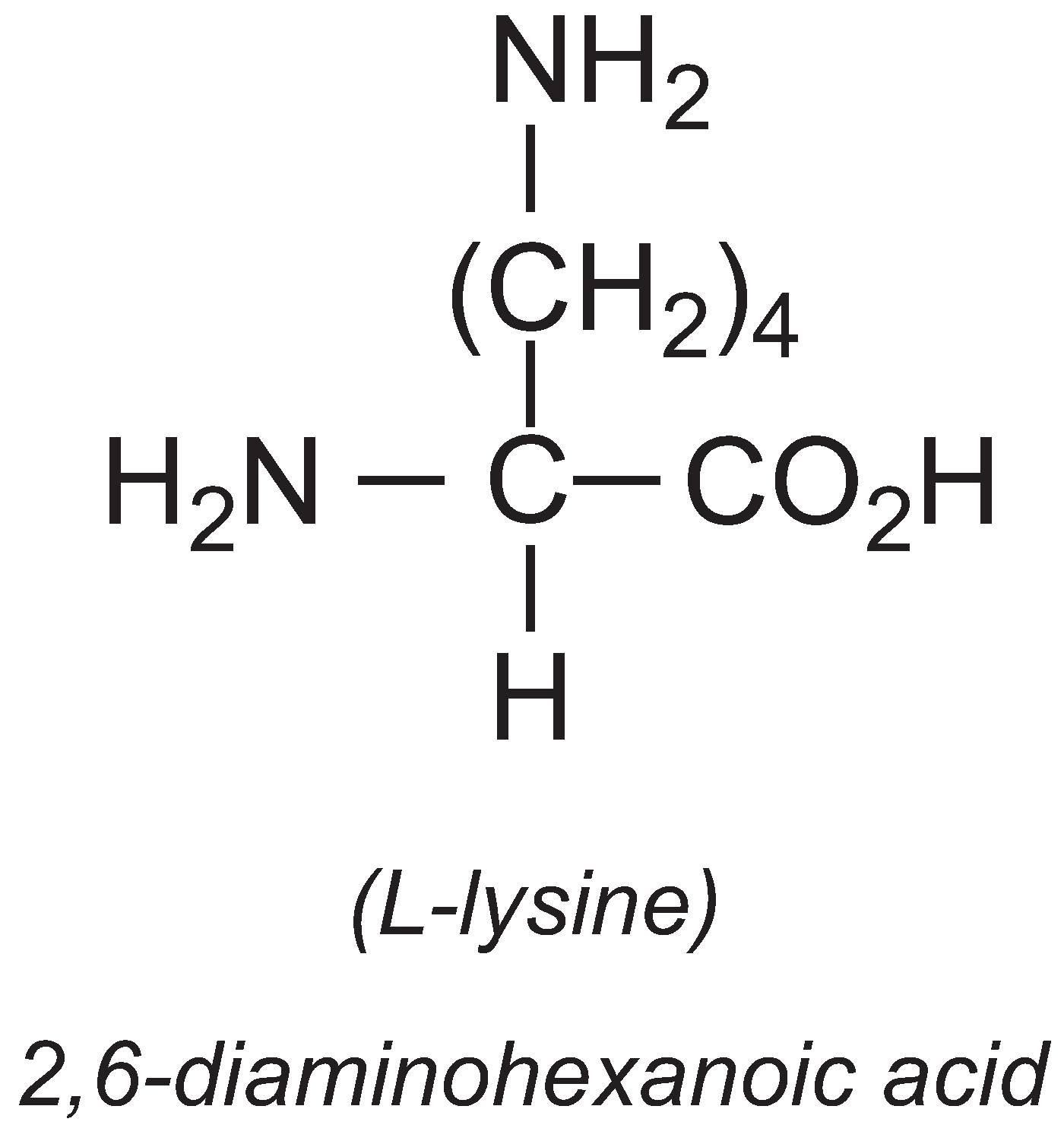
The annual production of L-lysine is about 1 million tonnes, with China producing 35%. It is an essential amino acid, meaning that most vertebrates cannot synthesise it. It is often deficient in livestock diets so the major use of L-lysine is in animal feed.
Date last amended: 18th March 2013
