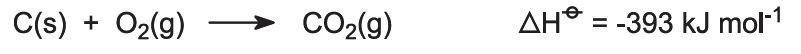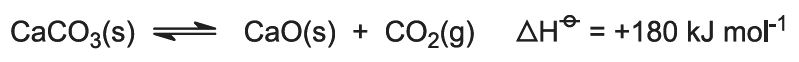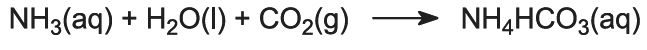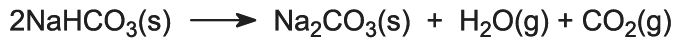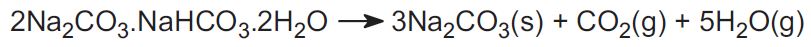Uses of sodium carbonate
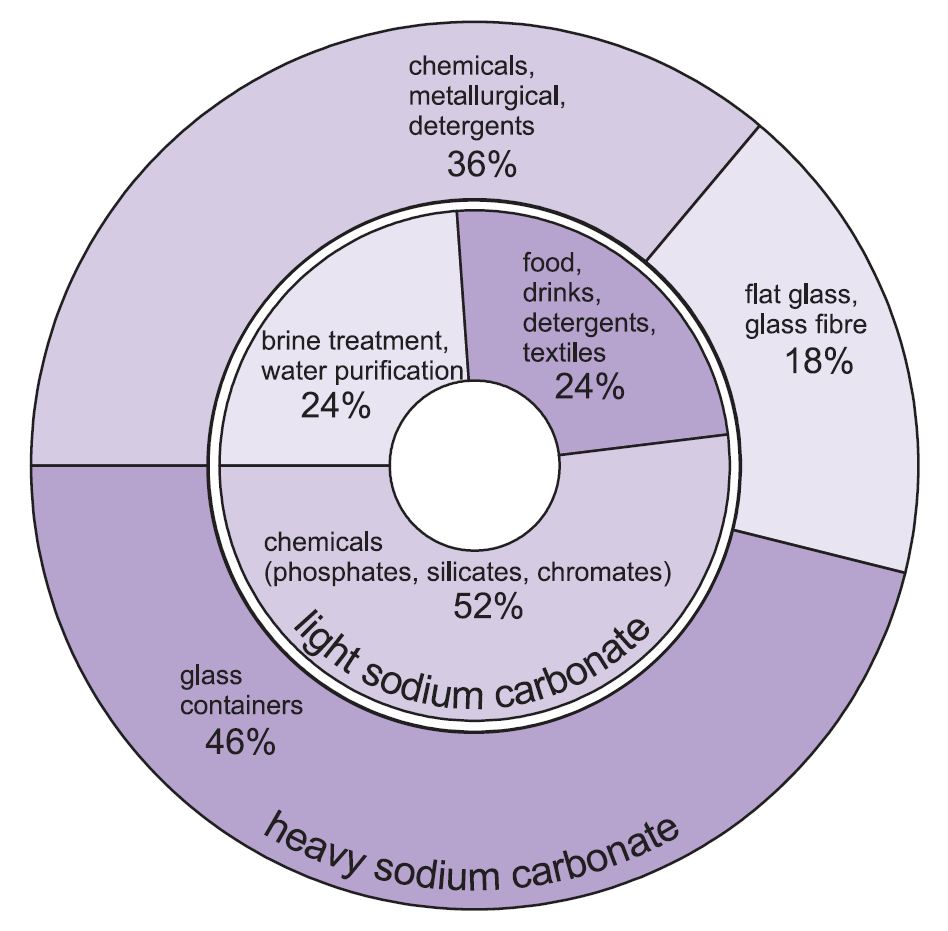
Two forms of sodium carbonate (soda ash) are used -heavy and light. The light form is obtained first and some is then converted into the heavy form. Light sodium carbonate contains less than 0.5% sodium chloride. Heavy sodium carbonate is obtained by hydrating light sodium carbonate to the monohydrate (Na2CO3.H2O) and then dehydrating it to give a product with an increased crystal size and density.
The two grades have different uses.
The major uses of heavy sodium carbonate are as a solid, particularly in making glass, where it is used as a flux in the melting of silica (sand).
The uses for light sodium carbonate are traditionally where the chemical is required in solution.
Figure 1 Uses of both heavy and light sodium carbonate.
Overall, about 50% of the total production of sodium carbonate is used to make glass, 18% to make other chemicals and 10% in soaps and detergents.
Annual production of sodium carbonate
China is the world's largest producer of sodium carbonate, accounting for 46% of world production and U.S. 23%. (IHS Markit, 2015)
| World | 52 million tonnes1,2 |
| China | 25 million tonnes3 |
| U.S. | 12 million tonnes1 |
| Europe | 11 million tonnes |
| Russia | 0.71 million tonnes4 |
Data from:
1. 2018 Elements of the Business of Chemistry, American Chemistry Council.
2. Of these, 14.7 tonnes are mined. The vast majority is mined in the U.S. (11.7 million tonnes) and Turkey (2 million tonnes). The rest is made from sodium chloride and calcium carbonate.
3. IHS Markit, 2015
4. Federal State Statistics Service: Russian Federation 2011
Manufacture of sodium carbonate
There are two main sources of sodium carbonate:
a) from salt and calcium carbonate (via the ammonia soda (Solvay) process)
b) from sodium carbonate and hydrogencarbonate ores (trona and nahcolite)
(a) From sodium chloride and calcium carbonate
The overall reaction can be regarded as between calcium carbonate and sodium chloride:
However, calcium carbonate is too insoluble to react with a solution of salt. Instead the product is obtained by a series of seven stages.
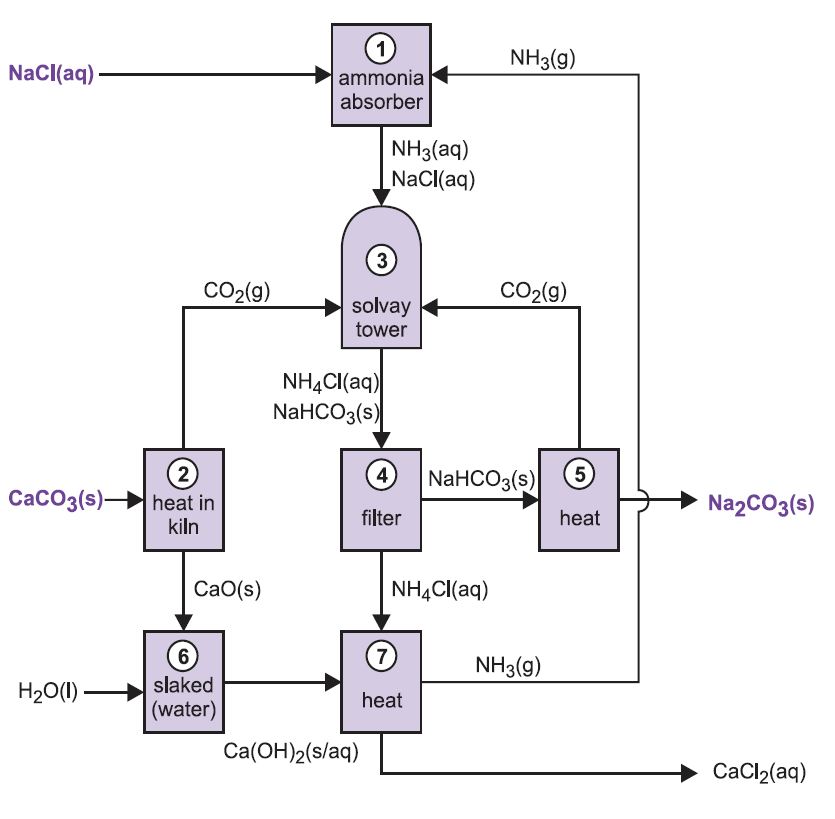
The process is known as the ammonia-soda process or the Solvay process, named after the Belgian industrial chemist who patented it in 186I.
The various stages of the Solvay process are interlinked as can be seen from the diagram and description below.
Figure 2 Different stages of the Solvay process.
(1) Ammoniation of brine
Ammonia gas is absorbed in concentrated brine to give a solution containing both sodium chloride and ammonia. Na+(aq), Cl-(aq), NH4+(aq), OH-(aq) ions and NH3(aq) are present.
(2) Formation of calcium oxide and carbon dioxide
Kilns are fed with a limestone/coke mixture (13:1 by mass). The coke burns in a counter-current of pre-heated air:
The heat of combustion raises the temperature of the kiln and the limestone decomposes:
The gas, containing approximately 40% carbon dioxide, is freed of lime dust and sent to the carbonating (Solvay) towers. The residue, calcium oxide, is used in ammonia recovery (see step 7 below).
(3) The Solvay Tower
This is the key stage in the process. The ammoniated brine from step (1) is passed down through the Solvay Tower while carbon dioxide from steps (2) and (5) is passed up it. The Solvay Tower is tall and contains a set of mushroom-shaped baffles to slow down and break up the liquid flow so that the carbon dioxide can be efficiently absorbed by the solution. Carbon dioxide, on dissolving, reacts with the dissolved ammonia to form ammonium hydrogencarbonate:
The solution now contains ions Na+(aq), Cl-(aq), NH4+(aq) and HCO3-(aq). Of the four substances which could be formed by different combinations of these ions, sodium hydrogencarbonate (NaHCO3) is the least soluble. It precipitates as a solid in the lower part of the tower, which is cooled. The net process is:
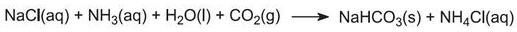
A suspension of solid sodium hydrogencarbonate in a solution of ammonium chloride is run out of the base of the tower.
(4) Separation of solid sodium hydrocarbonate
The suspension is filtered to separate the solid sodium hydrogencarbonate from the ammonium chloride solution, which is then used in stage (7).
(5) Formation of sodium carbonate
The sodium hydrogencarbonate is heated in rotating ovens at 450 K so that it decomposes to sodium carbonate, water and carbon dioxide:
The carbon dioxide is sent back to the Solvay Tower for use in step (3). The product of the process, anhydrous sodium carbonate, is obtained as a fine white powder known as light sodium carbonate.
(6) Formation of calcium hydroxide
The last two stages, (6) and (7), are concerned with the regeneration of ammonia from ammonium chloride (made in step 3). The quicklime from step (2) is slaked with excess water giving milk of lime:
(7) Regeneration of ammonia
This calcium hydroxide suspension is mixed with the ammonium chloride solution left from step (4) and heated:
The ammonia is thus recovered, and sent back to step (1). Calcium chloride is the only by-product of the whole process.
The overall process is an elegant one. In theory, the only raw materials are limestone and brine. Inevitably, there are losses of ammonia, and these are made up for by addition of extra supplies, as required in step (1).
(b) From trona and nahcolite ores
The Solvay process is not used in the US. Instead, the industry uses two ores and this accounts for about 30% of the world's production. One is trona, found in vast amounts in Wyoming. Trona has the formula:
The ore is mined as a solid and heated to drive off carbon dioxide, to yield sodium carbonate:
The other ore is nahcolite which is sodium hydrogencarbonate. On heating it forms sodium carbonate.
Date last amended: 27th November 2018