.jpg) There are few better examples of improvements in the manufacture of chemicals in recent years than that of ethanoic acid (acetic acid). Up to the last few years much was manufactured by the non-catalytic oxidation of naphtha which gave large quantities of side-products. It is now usually manufactured from methanol with yields of over 99%.
There are few better examples of improvements in the manufacture of chemicals in recent years than that of ethanoic acid (acetic acid). Up to the last few years much was manufactured by the non-catalytic oxidation of naphtha which gave large quantities of side-products. It is now usually manufactured from methanol with yields of over 99%.
Uses ethanoic acid (acetic acid)
Much of the ethanoic acid produced is converted into ethenyl ethanoate (vinyl acetate) which is the monomer for poly(ethenyl ethanoate) and ethanoic anhydride (acetic anhydride) which is used principally to make cellulose ethanoate.
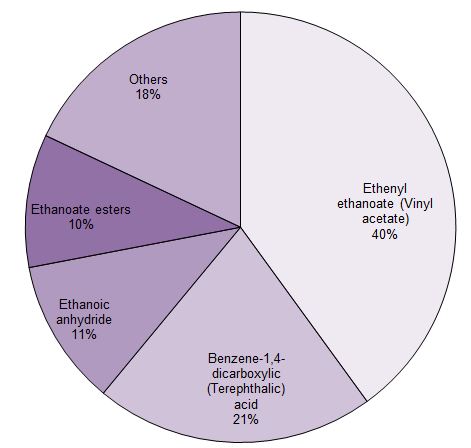
Figure 1 The uses of ethanoic acid.
Data from Methanol Market Services Asia, 2014. Data (estimated) for 2013
The acid itself is the solvent for the liquid phase oxidation of 1,4-dimethylbenzene (p-xylene), leading to the production of polyesters.
| Figure 2 The road surface on the Pont de Normandie, in Northern France, has been made from co-polymers, including one using ethene and ethenyl ethanoate. The ester is produced from ethanoic acid. By kind permission of Arkema. |
.jpg) |
Annual production of ethanoic acid (acetic acid)
| World | 12.1 million tonnes1 |
| China | 4.4 million tonnes2 |
| Rest of Asia | 3.1 million tonnes2 |
| U.S. | 2.8 million tonnes3 |
| Europe | 1.8 million tonnes2 |
Data from:
1. Latest figures for Global are 12.1 million tonnes (2014) and 16.3 million tonnes estimated for 2018. MarketsandMarkets, 2016
2. Extrapolated from Transparency Market Research, 2015
3. 2015 Business of Chemistry Council, 2016
The newly manufactured acid is sometimes known as virgin ethanoic acid (virgin acetic acid). About another 2 million tonnes is recovered annually, particularly from the process leading to polyesters.
Manufacture of ethanoic acid (acetic acid)
The main route is from methanol.
Methanol and carbon monoxide, both formed via synthesis gas are reacted together in the liquid phase, with some water to keep the catalyst in solution, at moderate temperatures of about 450 K and a pressure of 30 atm:
.jpg)
A rhodium/iodine based catalyst system was first used. The catalyst has recently been improved, based on iridium in place of rhodium, the Cativa process. Yields of more than 99% ethanoic acid are obtained.
.jpg)
Figure 3 The manufacture of ethanoic acid using the Cativa process.
1 The reactor. 2 Distillation column to remove methanol, water and carbon monoxide.
Propionic acid is removed on further distillation.
By kind permission of BP.
The main reason for the improvement is that much less water is needed to keep the iridium complex in solution compared with the amount needed with the rhodium complex. Thus by reducing the consumption of energy on distillation the cost of purifying the product falls by an estimated 30%. This means that greenhouse gas emissions are also reduced.
The two reactants, methanol and carbon monoxide are mixed with iridium(IV) chloride and hydrochloric acid (H2IrCl6) in ethanoic acid which acts as a solvent. Iodomethane, CH3I, is also added which forms a complex ion, [Ir(CO)2I3CH3]-, which is considered to be the crucial catalytic species. A ruthenium compound is added which acts as a promoter.
Date last amended: 26th October 2016
