.jpg) Although some bromine compounds can be very harmful to the environment (for example bromomethane leads to depletion of ozone in the stratosphere), they are also extremely important in the manufacture of many indispensable compounds.
Although some bromine compounds can be very harmful to the environment (for example bromomethane leads to depletion of ozone in the stratosphere), they are also extremely important in the manufacture of many indispensable compounds.
Uses of bromine
Several important flame retardants, such as tetrabromobisphenol A, are derived from bromine and bromine compounds although there are concerns about the effectiveness of some of them. The flame retardants are, for example, either bonded (chemically) or incorporated physically, into plastics. This is particularly important when plastics are used where there are fire hazards.
Bromine is also used in the manufacture of biocides, such as biobrom (2,2-dibromo-3-nitrilopropionamide). This is used extensively in treating water
Concentrated solutions of calcium bromide and magnesium bromide (collectively known as 'clear brines') are used in oil drilling. They are very dense solutions (ca 1.8 kg m-3) and when poured into the drilling hole, sink through the water to the bottom. They help to lubricate the drill, increasing its capacity to drill deeper. They also help to disperse the solids being drilled out.
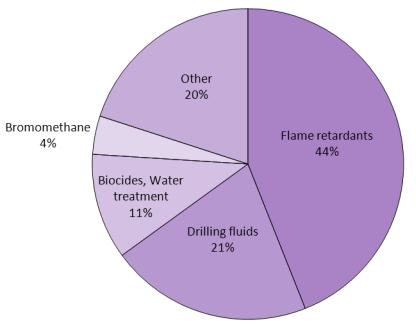
Figure 1 The uses of bromine.
While Figure 1 shows data for the worldwide use of bromine, the proportion varies country by country. For example in the US, less (45%) is used to make flame retardants and more (21%) as clear brines for drilling.
A further use of bromine is in making bromomethane, used as a fumigant against pests, and to clean soils before planting. However, this is decreasing in importance as there are international agreements to reduce its use as, like CFCs, it is considered to lead to depletion of ozone when it is transported into the stratosphere. It is now used under strictly limited conditions in developed countries but is allowed to be used until 2015 by developing countries.
Annual production of bromine
| World | 390 000 tonnes |
| Israel | 150 000 tonnes |
| Jordan | 100 000 tonnes |
| China | 100 000 tonnes |
| Japan | 30 000 tonnes |
| Ukraine | 3 500 tonnes |
Data from:
U.S. Geological Survey, Mineral Commodity Summaries, 2016.
The figure for the United States has been witheld. The most recent figures available show that they were similar to the production of Israel.
Manufacture of bromine
Bromine is obtained by oxidation of bromide ions:
.jpg)
The process occurs in two identifiable stages:
a) oxidation of bromide ions to bromine
b) purification of bromine
(a) Oxidation of bromide ions to bromine
Chlorine and water are pumped up a tower, down which hot brine, rich in bromide ions, is flowing.
Bromine is liberated from the solution by oxidation of the bromide ions by the chlorine gas:
.jpg)
(b) Purification of bromine
The crude bromine, containing water, chlorine and organic matter, is purified by distillation. The bromine in the solution is 'stripped out' with steam. In one process (used in the US), steam is pumped through the liquid, under reduced pressure, until it is boiling. The bromine is collected together with the condensed steam and separated into two layers as bromine is only slightly soluble in water. In Israel, the steam distillation is carried out at atmospheric pressure. The bromine is further purified by distillation, and then dried with concentrated sulfuric acid. About 95% of the bromine is recovered from the brine.
Seawater contains bromide ions, but in much smaller quantity than chloride ions, about 65 ppm (parts per million). Indeed 20 000 tonnes of seawater needs to be processed to produce 1 tonne of bromine.
However, there are sources available with much higher concentrations of bromide ions. Examples are the salt deposits in Arkansas, in the US, where concentrations of bromide ions range from 4000-6000 ppm and the Dead Sea in Israel, where the concentration is even greater, 6000-12 000 ppm.
Date last amended: 6th October 2016
