Colorants are used in many industries - to colour clothes, paints, plastics, photographs, prints, and ceramics. Colorants are also now being used in novel applications and are termed functional (high technology) as they are not just included in the product for aesthetic reasons but for specific purposes, for example in surgery.
Colorants can be either dyes or pigments. Dyes are soluble coloured organic compounds that are usually applied to textiles from a solution in water. They are designed to bond strongly to the polymer molecules that make up the textile fibre.
Pigments are insoluble compounds used in paints, printing inks, ceramics and plastics. They are applied by using a dispersion in a suitable medium. Most pigments used are also organic compounds.
Manipulating the colour and application of dyes
This section considers some of the chemistry behind the colour of dyes and how the target material, for example a fibre, influences the method of dyeing and the dye used.
A dye in solution is coloured because of the selective absorption of certain wavelengths of light by specific bonds in the molecule. The light that is transmitted is seen by the observer and appears coloured because some of the wavelengths of the visible spectrum are now missing.

Figure 1 Mauveine was the first ever synthetic dye. It was produced accidentally by William Perkin in 1856 who was trying to synthesize quinine. It became particularly popular when Queen Victoria wore a silk gown dyed with mauveine at the Royal Exhibition of 1862 in London.
By kind permission of the Society of Dyers and Colourists.
The absorption of visible light energy by the compound promotes electrons in the molecule from a low energy state, the ground state, to a higher energy state, the excited state. The molecule is said to have undergone an electronic transition during this excitation process. Particular excitation energies correspond to particular wavelengths of visible light.
It is a n electron (an electron in a double or triple bond) that is promoted to the excited state. Even less energy required for this transition if alternate single and double bonds (i.e. conjugated double bonds) exist in the same molecule. The excitation of the electron is made even easier by the presence of aromatic rings because of th enhanced delocalisation of the n electrons.
By altering the structure of the compound, colour chemists can alter the wavelength of visible light absorbed and therefore the colour of the compound.
The molecules of most coloured organic compounds contain two parts:
(i) a single aryl (aromatic) ring such as benzene or a benzene ring with a substituent. Alternatively there may be a fused ring system such a naphthalene (two rings fused together) or anthracene (three rings fused together).
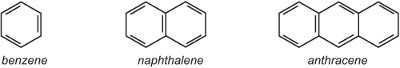
Where the rings join, they share two carbon atoms and thus naphthalene with two rings has 10 carbon atoms, not 12. Similarly, anthracene has 14 carbon atoms rather than 18. As naphthalene and anthracene contain delocalised electrons over all the rings it is inappropriate to use the delocalised symbol which is used for benzene in the other units, for that would indicate two or three separate delocalised systems. Thus in this unit, Kekule structures are used.
(ii) an extensive conjugated double bond system containing unsaturated groups, known as chromophores, such as:
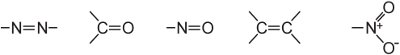
The intensity of colour can be increased in a dye molecule by addition of substituents containing lone pairs of electrons to the aryl ring such as:
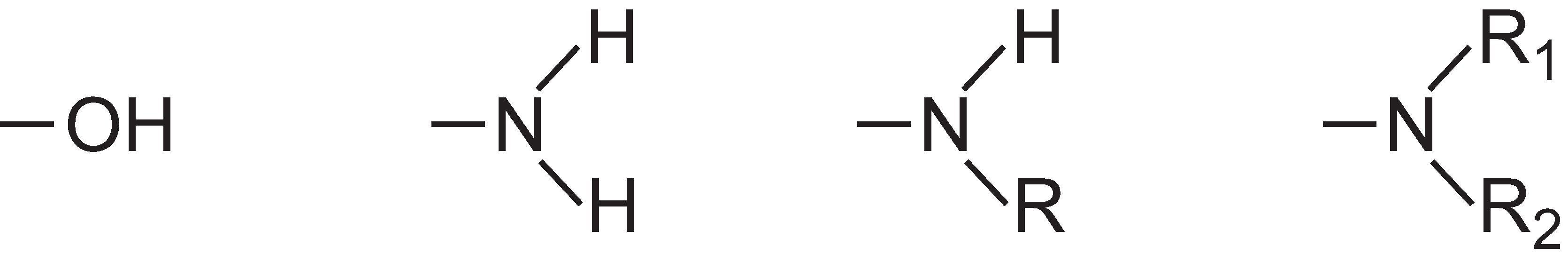
These groups are known as auxochromes.
Sometimes the entire structure of the colorant is called the chromogen.
To make the colorant of importance industrially, colour chemists must also be able to alter the compound's solubility, and groups may be included to make the colorant soluble in water. Examples include the sulfonic acid group, -SO3H, or the carboxylic acid group, -COOH, or more usually, the sodium salt of these acids, -SO3-Na+ and -COO-Na+, respectively.
Another key concern of chemists developing dyes is to enhance its reactivity with the object that they want to colour, for example the molecules of the fibre. This is discussed below and examples are given throughout the unit.
The coloration of textiles
The chemical nature of a dye is determined by the chemical and physical properties of the fibres of the textile to be coloured. The four main types of fibres (Table 1) are protein, cellulosic, regenerated (based on cellulose or derivatives) and synthetic.
| Natural fibres | Man made fibres | ||
|---|---|---|---|
| Protein | Cellulose | Regenerated | Synthetic |
| Wool | Cotton | Viscose rayon | Polyamides |
| Silk | Linen | Celulose ethanoates | Polyesters |
| Mohair | Ramie | Acrylics | |
| Cashmere | |||
| The term regenerated is used when a natural polymer has been treated chemically to form another polymer. For example, natural cellulose from plants, when treated with ethanoic anhydride (acetic anhydride), produces a polymer, cellulose ethanoate, which is rayon. |
|||
Table 1 Classification of textile fibres.
During the process of dyeing a textile, the dye is distributed between the two phases, the solid fibre phase and the aqueous phase, and at the end of the dyeing process the solution is depleted and most of the dye is associated with the fibre. Once the dye molecules penetrate the fibre there is immediate interaction between the two components, which prevents desorption of the dye molecules back into solution. The type of interaction, whether physical or chemical, will depend on the groups on the dye molecules and in the fibre chains (Table 2).
| Bond type | Approximate relative strength |
|---|---|
| covalent | 30.0 |
| ionic | 7.0 |
| hydrogen | 3.0 |
| other intermolecular | 1.0 |
Table 2 Approximate relative strengths of bonding between a dye and a fabric.

Figure 2 Before a colorant is used, its light fastness must be determined. These
racks, situated on the North-East coast of Australia, are used for many tests of
weathering, amongst them being colour fastness. The position of the racks can
be altered but in the photo, they are at an angle of 45° to the horizontal.
By kind permission of the Allunga Exposure Laboratory.
The colour fastness of a coloured textile is defined as its resistance to change when subjected to a particular set of conditions. The dye should not be affected greatly by sunlight (light fastness), heat when the fabric is ironed (heat fastness), perspiration (perspiration fastness) and when washed (wash fastness).
Classification of colorants
The Colour Index International, produced by the Society of Dyers and Colourists, in Bradford, is a comprehensive list of known commercial dyes and pigments and is updated regularly. Each colorant is given a Colour Index (C.I.) Name and Number. For example:
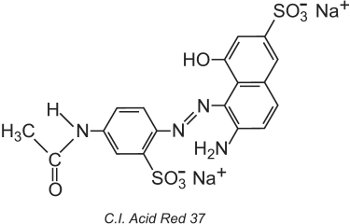
All the colorants in the list have been classified by their chemical structure and by their method of application.
Classification of colorants by their chemical structure
The Colour Index assigns dyes of known structure to one of 25 structural classes according to chemical type. Amongst the most important are:
a) azo dyes
b) anthraquinone dyes
c) phthalocyanines
(a) Azo dyes
The azo dyes constitute the largest chemical class, containing at least 66% of all colorants. The characteristic feature is the presence in the structures of one or more azo groups,

together with hydroxyl groups, amine and substituted amine groups as auxochromes.
Aromatic azo compounds are produced from aromatic amines via the corresponding diazonium salt.
A diazonium salt is formed when an aromatic amine is treated with nitrous (nitric(III)) acid. The nitrous acid is formed in situ by adding dilute hydrochloric acid to a cool solution of sodium nitrite at ca 278 K. In the following example, a solution of benzenediazonium chloride has been formed from phenylamine (aniline), the simplest aromatic amine:

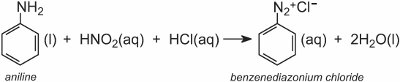
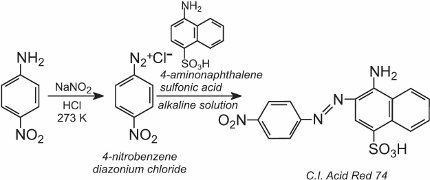
A solution of another compound such as another aromatic amine or a phenol is then added to the cool solution and produces an azo compound which is coloured. One example is the formation of a red dye when an aqueous solution of 4-aminonaphthalenesulfonic acid (naphthionic acid) is added to a solution of 4-nitrobenzenediazonium chloride to form C.I. Acid Red 74:
Azobenzene is the chromophore of these azo dyes,
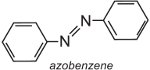
and the colour of the molecule can be modified and the intensity of colour increased by varying the auxochromes (Table 3).
| Structure | Colour observed |
|---|---|
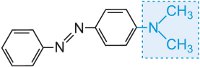 |
yellow-green |
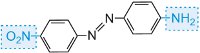 |
yellow |
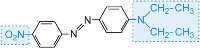 |
red |
 |
blue |
 |
Table 3 The molecular structures of some azo dyes showing the auxochromes.
Some azo dyes, those containing a hydroxy group ortho (or para) to the azo group, for example, C.I. Acid Orange 7, exhibit tautomerism, a process in which the molecule exists as two or more different structures in equilibrium. The hydrogen atom on the hydroxyl group is able to migrate to the nitrogen atom of the azo group and vice versa:
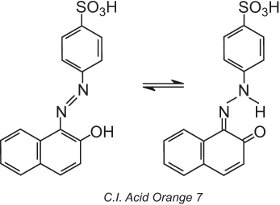
This type of tautomerism involves an equilibrium between a hydroxyazo tautomer and a ketohydrazone tautomer, although the ketohydrazone tautomer generally dominates and the colour observed is of longer wavelength (a bathochromic shift).
(b) Anthraquinone dyes
Anthraquinone dyes account for about 15% of colorants and have structures based on quinones. The simplest quinone is benzoquinone, which has two isomers:
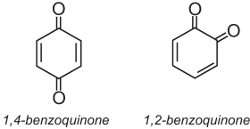
Anthraquinone, the simplest of the anthraquinones, is based on anthracene:
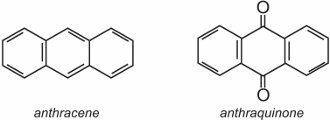
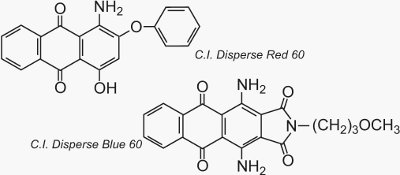
Two well known examples of anthraquinones which are used as dyes are C.I. Disperse Red 60 and C.I. Disperse Blue 60:
(c) Phthalocyanines
Phthalocyanines are essentially made up of four molecules of isoindole:
These molecules are connected to each other in a phthalocyanine by nitrogen atoms. The structure of phthalocyanine is:
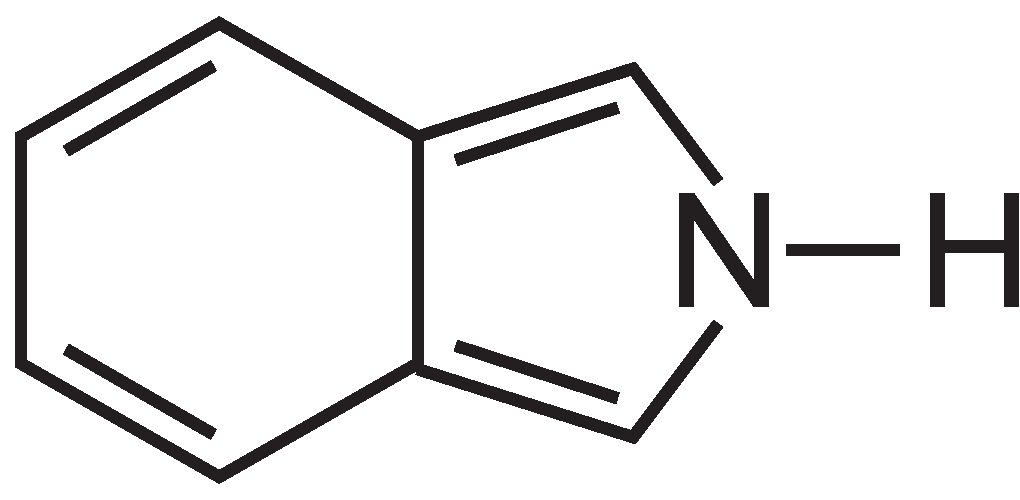
These molecules are connected to each other in a phthalocyanine by nitrogen atoms. The structure of phthalocyanine is:
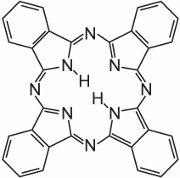
Phthalocyanines coordinate with metal atoms. The most important, contributing about 2% of all colorants, are the copper phthalocyanines, used for their brilliant blue and green colours. An example is C.I. Direct Blue 86:
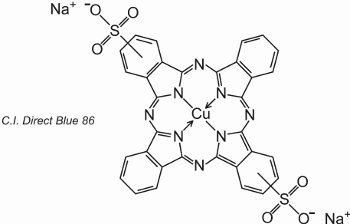
The sulfonic acid groups assist the solubility of the dye in water.
The formula indicates that the sulfonic acid groups can be at different positions on the aromatic rings.
Classification of colorants by methods of application
Classification by the method of application is important to the textile dyer applying the dye to produce the colour required. To obtain the required shade the dyer usually has to make mixtures of dyes and must ensure that these are compatible.
The basic features that control dye transfer from solution to fibre are:
- the pH of the solution in the dyebath (for acid and basic dyes)
- an electrolyte (a solution of sodium sulfate or chloride)
- the temperature (within the range of ambient to 400 K)
- chemicals, known as dispersing agents, that produce a stable aqueous dispersion of dyes of very low solubility
Table 4 lists the dyes under their technological names that indicate how they are applied, along with the fibres to which they are applied.
| Dye | Fibre |
|---|---|
| Group 1 | |
| Acid | Wool and other protein fibres, polyamides |
| Metal-complex | Wool and other protein fibres, polyamides |
| Direct | Cotton, linen, viscose |
| Basic | Acrylic |
| Disperse | Polyesters, polyamides, ethanoates |
| Group 2 | |
| Reactive | Cotton, linen, viscose, wool, silk |
| Vat | Cotton, linen, viscose |
| Sulfur | Cotton, linen |
Table 4 Technological classification of dyes.
Group 1 dyes
Dyes in this group are characterized by their solubility in water. Consequently they are not particularly fast on washing. The method of application involves only a single stage process.
(i) Acid dyes
The important chemical types are azo, anthraquinone and phthalocyanine, which cover the whole of the visible spectrum and thus give a complete colour range. These dyes are soluble in water giving anionic species. They are usually applied at ca 373 K. Whereas wool and other protein fibres degrade readily above this temperature, polyamide fibres (for example, the nylons) can be treated at 393 K without any harm coming to them (Table 4).
The pH chosen for the solution in the dyebath depends on the individual properties of the dyes. The lower values are obtained by adding sulfuric acid and higher values by adding solutions of ethanoic acid and ammonium sulfate or ammonium ethanoate. Sodium sulfate may be added to control the diffusion of the dye anions in the fibre structure.
By the very nature of the dye structure, ionic bonds, hydrogen bonds and other intermolecular interactions (Table 2) will form between the dye and the fibre thus making the dyes fast. An example of a typical acid dye is C.I. Acid Red 73:
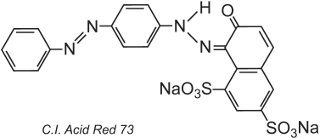
One of the azo groups in this tautomer is present as the ketohydrazone form.
(ii) Metal-complex dyes
The chemical types are azo and anthraquinone giving a complete colour range. However they are duller than the acid dyes because of the presence within the dye structure of a metal atom. Chromium salts are often used although cobalt and nickel salts are also favoured.
The metal atom forms a coordination complex with two molecules of a monoazo compound containing hydroxyl, carboxyl or amino groups in the 2,2' positions relative to the azo group. These compounds are called '1:2 metal complex' dyes. An example is C.I. Acid Violet 78:
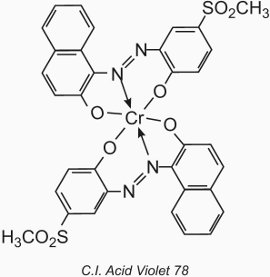
Their application to wool is similar to that for acid dyes, but the pH value is restricted to the range of 4.5 to 6.0 (Table 4).
(iii) Direct dyes
Direct dyes are particularly useful for dyeing fibres made from cellulose (Tables 1 and 4).
They are synthesized with sulfonic acid groups to give them solubility in water, dissociating to give sodium cations and the anionic dye species. They are also designed so that they are as linear and planar in structure as possible. This allows the dye to be attached to the cellulosic chains in the fibre, often via intermolecular (including hydrogen) bonding.
They are applied in the dyebath in aqueous solution which contains sodium chloride. The salt reduces the electrical forces of repulsion between the negative charge on the fibre surface and the anionic dye species.
Most of the direct dyes are azo compounds, often containing two or three azo groups. Examples include C.I. Direct Orange 25 which has -OH, -NHCO- and -N=N-groups all of which have the potential to form hydrogen bonds with the hydroxyl groups in cellulose:
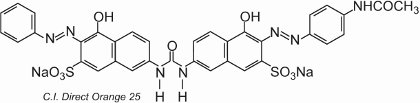
The colorant exhibits tautomerism, as there are two hydroxyl groups ortho to the azo groups. One of the tautomers in equilibrium with this form is
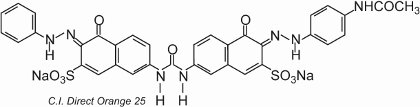
where there are two ketohydrazone groups.
Another example, C.I. Direct Blue 71, has three azo groups, one of which is present as the ketohydrazone tautomer:
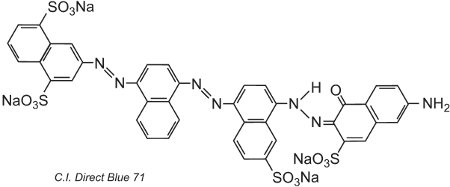
(iv) Basic dyes
Basic dyes were amongst the earliest synthetic dyes. Indeed Mauveine is a basic dye. The chromophore is present as a cation and they are used nowadays in dyeing acrylic fibres (usually a co-polymer with propenonitrile (acrylonitrile) and a small amount of a co-monomer which contain sulfonate, -SO3-, and carboxylate, -CO2-, groups). These are ion-ion interactions (Tables 2 and 4).
There are about 100 basic (cationic) dyes whose colours span reds, yellows and blues, with bright strong shades. Some are based on the azo and anthraquinone chromophore systems. Many are also based on arylcarbonium ions. Examples include C.I. Basic Green 4 (known as Malachite Green) and C.I. Basic Red 9.
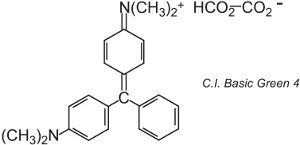 |
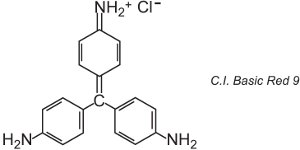 |
These are both triarylmethanes, a group of dyes which with relatively small changes in structure produce a range of red, green and violet hues.
Others, known as polymethine dyes (they contain one or more -CH= groups) are also used. They owe their colour to the presence of a conjugated system. An example of such a dye is C.I. Basic Yellow 28 which is a diazacyanine:
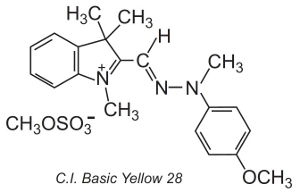
The dyes are often applied in a solution of an electrolyte, which controls the rate of diffusion in the fibre structure, at temperatures of about 370 K.
(v) Disperse dyes
These dyes are essentially hydrophobic and are almost insoluble in water. However they have affinity for hydrophobic fibres, for example polyesters, and are applied as very fine dispersions in water (Table 4).
Most disperse dyes are azo compounds and can give colours across the spectrum. Some are anthraquinone based dyes for reds, violets, blues and greens.
Polyester fibres can be dyed at 400 K under pressure, allowing the use of larger molecular size dye structures which achieve better fastness, for example:
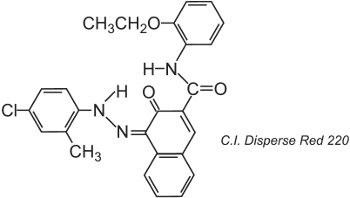
The structure shown is the ketohydrazone tautomer.
Group 2 dyes
Although the dyes in this group are applied by a two stage process (which contrasts with the single stage process for Group 1 dyes), the dyes have advantages, in particular with fastness.
i) Reactive dyes
Reactive dyes are of outstanding importance for the dyeing of cotton, enabling bright intense coloration with high fastness. Approximately 95% of reactive dyes are azo dyes covering all the range of colours. Blues and greens are also provided by anthraquinone and phthalocyanine structures.
As the name of these dyes suggests, they react with the fibre, whether cellulosic (cotton) or protein (wool) to form covalent bonds (Table 4). The two stages, first dyeing, then reaction, can take place separately or simultaneously. The characteristic structural feature is the presence of one or more reactive groups. Typically the dyes are depicted as
D-B-RG
where D is the chromogen, B a bridging group and RG the reactive group.
The most important reactive groups are the chlorinated triazines and vinylsulfones.
One of the three isomers of the simplest triazine is:
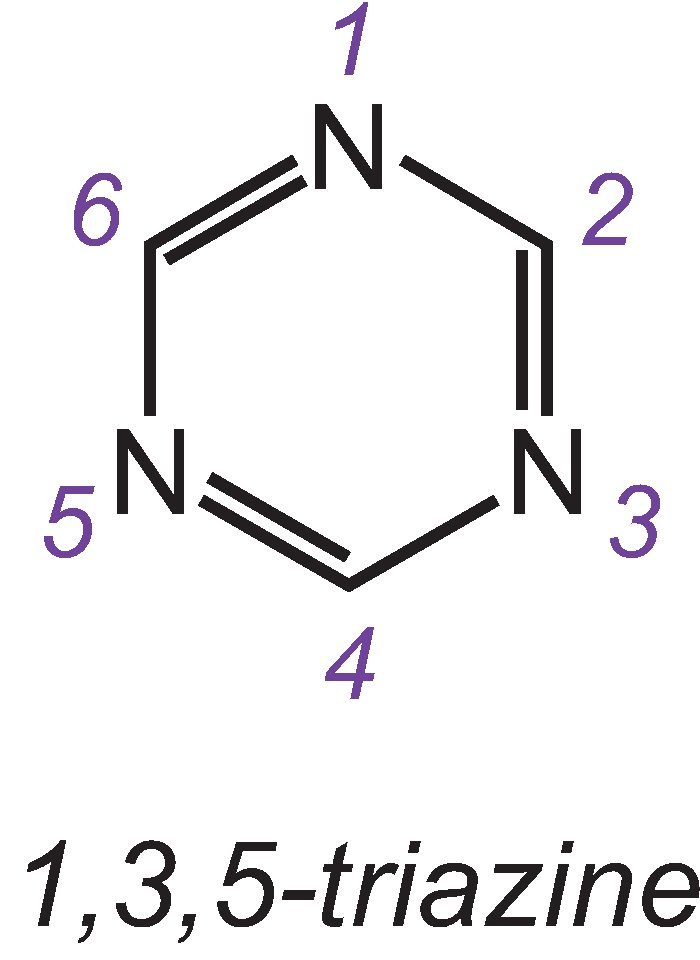
An example of a dye with a dichlorotriazine group is C.I. Reactive Blue 109:
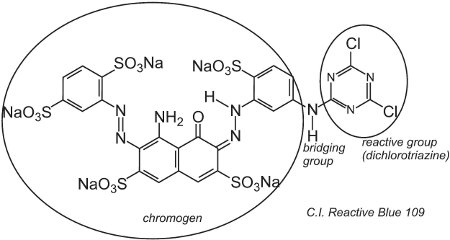
The reaction between the -OH groups of the cellulose in the fibre and the -C-Cl groups in the chlorotriazine is by a (nucleophilic) substitution reaction to form covalent bonds.
An ethenyl (vinyl) sulfone contains the CH2=CHSO2-group and the simplest is diethenylsulfone (divinylsulfone). The sulfone group can be seen in C.I. Reactive Blue 19:
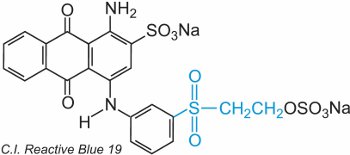
In this example, there is no bridging group.
The dye reacts with cellulose by addition to the sulfur-oxygen double bond.
Reactive dyes, in the aqueous solution, can undergo hydrolysis of the sulfone making it unreactive to the cellulose. This means that unreacted dye, if not washed off properly, will remain on the surface of the fabric giving an apparent colour that will wash out over time. To reduce this problem, dyes have been designed with two different reactive groups of differing reactivity. These dyes offer improved fastness because if one of the groups is hydrolyzed in solution, the other one will react with the hydroxyl groups in the fabric. The first of these included both a chlorotriazine and vinylsulfone groups and an example is C.I. Reactive Red 194:
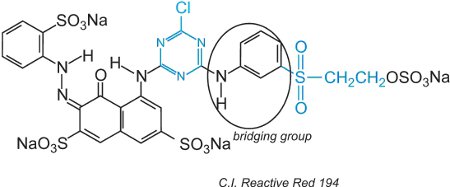
Besides the two different reactive groups, there is a chromogen and a bridging group.
All the reactive dyes have a relatively small molecular size and they also have two or more sulfonic acid groups in the chromogen, leading to a high solubility in water. A proportion of dye species (anionic) does not react with the fibre and is hydrolyzed and the product must be removed by washing.
 |
|
Figure 3 Jeans are dyed with indigo and a variety of sulfur dyes, the choice depending on the colour desired. |
(ii) Vat dyes
Approximately 80% of vat dyes belong to the anthraquinone chemical class of dyes and cover the full colour range. One type, the indigoid dyes, includes indigo:
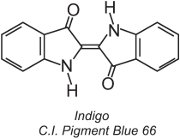
All vat dyes are insoluble in water. To apply them to a fibre, for example cotton, they are placed in an alkaline solution (Table 4). The insoluble dye is reduced to form a colourless (leuco) anion which is soluble and possesses affinity for the fibre. This is then adsorbed by the fibre, sometimes in the presence of sodium chloride, conditions similar to that for direct dyes. After the dyeing process the original insoluble parent dye is regenerated within the fibre by oxidation, usually using a solution of hydrogen peroxide or simply air:
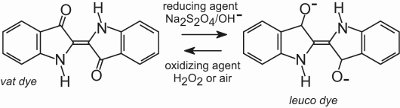
The dyes are insoluble within the fibre structure and therefore have good wash fastness and they also possess high light fastness.
(iii) Sulfur dyes
Sulfur dyes like vat dyes are applied to textiles (cellulose, Table 4) as a soluble anionic form and then oxidized into the insoluble form.
C.I. Sulphur Black 1 and C.I. Sulphur Blue 7 are amongst the most widely used sulfur dyes. Like other sulfur dyes, their structures are variable and largely unknown. They provide a range of blacks, browns and dull blues. They are however much cheaper than vat dyes to produce because their preparation by heating various organic compounds with sulfur, is simple.
Pigments
Pigments are used in the coloration of paints, printing inks, ceramics and plastics. They can be used on a much wider variety of substances than dyes because they are not reliant on water solubility for their application. A pigment is a finely divided solid which is essentially insoluble in its application medium. In most cases the pigment is added to a liquid medium e.g. wet paint or a molten thermoplastic. The medium is then allowed to solidify by solvent evaporation or cooling and so the pigment molecules become fixed mechanically in the solid state.
The chromophores used in pigments are usually the same as those used in dyes but the pigments are large molecules and do not have solubilising groups. They contain groups that form intermolecular bonds that help to reduce solubilities. The larger the molecule, the more opaque the pigment.

| Figures 4 and 5 The red and yellow colorants are azo pigments (C.I. Pigment Red 57 and C.I. Pigment Yellow 13). The blue is the pigment copper phthalocyanine. By kind permission of BASF. |
 |
Organic pigments generally produce a higher intensity and brightness of colour than inorganic pigments such as chrome yellow (lead(II) chromate(VI)).
Organic pigments exhibit a range of fastness properties that are dependent on the molecular structure and the nature of the intramolecular association in the solid state. An increase in the molecular size of a pigment generally decreases the solubility of the pigment. Also many pigments have the amide group (-NHCO-) incorporated which further decreases its solubility as the molecules are held together in large structures by hydrogen bonding (between the N-H group in one molecule and a C=O group in another).
Many organic pigments are based on azo chemistry and dominate the yellow, orange and red shade areas. An example of a simple monoazo pigment is C.I. Pigment Yellow 1:
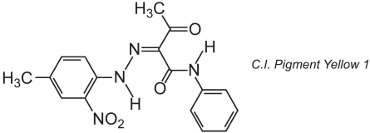
This form is the ketohydrazone tautomer.
Copper phthalocyanines provide the majority of blue and green pigments. They are structurally complex but are relatively inexpensive to make. They provide excellent resistance to light, heat, acids and alkalis.
An example is C.I. Pigment Blue 15:
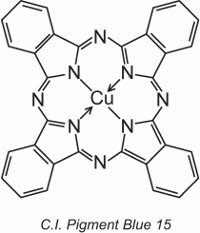
Earlier in the unit, the structure of a dye, C.I. Direct Blue 86, was displayed and it can be seen how the sulfonic acid groups in that structure transform a pigment into a dye.
Functional (high technology) dyes and pigments
Functional dyes and pigments are produced in small volumes compared to compounds used for dyeing textiles. However, they are the subject of much research and interest and are being developed for a variety of purposes. Some of these are illustrated below.
(a) Liquid crystal displays
Liquid crystals have played an important part in our lives for many years in various forms of information displays e.g. calculators. Initially they could only display differences between light and dark. It was found that by using dyes this contrast could be increased and coloured screens produced. They have now largely replaced the traditional display technologies of light emitting diodes and cathode ray tubes. The dyes used have been specifically designed to change orientation with the liquid crystal molecules and therefore offer a higher intensity of colour. These dyes are said to exhibit dichroism.
(b) Laser dyes
The term laser is an acronym referring to light amplification by stimulated emission of radiation.
Commonly inorganic lasers were used but only had the ability to produce radiation at a few selected wavelengths and in very narrow bands. The use of dyes has allowed for the production of light throughout the spectrum from wavelengths of 320 to 1200 nm. The application of dye lasers includes communication technology, and microsurgery.
(c) Ink jet printing
Ink jet printing is a non-impact technique to produce images by directing small droplets of ink, ideally under computer control, in rapid succession onto a substrate. It has found many applications. Because of the size requirements for the droplets to be able to achieve good definition the use of dyes has been favoured over pigments. Droplets are smaller (pigments tend to block the nozzles) and aqueous solubility reduces the environmental impact and keeps the price low. Early dyes were those already used in other industries but were characterized by poor water fastness. This has led to the development of specific dyes and unique fluid systems. These dyes are designed to be soluble in slightly alkaline systems (pH 7.5 to 10) which are made insoluble by the slightly acidic conditions (pH 4.5 to 6.5) on the paper or other substrate. This technology is having a great impact on high volume industrial printing for packaging, textiles, wallcoverings and advertising displays.
(d) Photodynamic therapy
This is a treatment for cancer that uses a combination of laser light, a photosensitizing compound (the dye) and molecular oxygen. The dye is administered to the patient intravenously and over time enters the cancerous cells. Irradiation of the cells with laser light can start their destruction.
The laser interacts with the dye and promotes it to its excited state. Through a complex process, excited (more reactive) oxygen molecules are produced which react with unsaturated centres in the proteins and lipids in the cell membrane. This method of treatment avoids the use of invasive surgery.
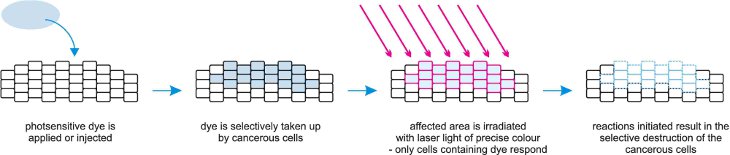
Figure 6 Illustrating the technique of photodynamic therapy.
Date last amended: 18th March 2013
