Biomass is anything made from living or recently dead plant or animal material. For thousands of years humans have harnessed natural sources of energy, such as wind power for sailing ships and windmills, and water power from fast-flowing rivers. We have also learned how to exploit biomass for fuel and even today many societies still depend on wood, peat and even cow dung for fuel. Dramatic change came with the discovery and use of fossil fuels, these being energy dense (giving lots of energy per mass of material) and easily transportable. It was coal that powered the Industrial Revolution and the invention of the steam engine that revolutionised transport. Suddenly people and goods could move about, unconstrained by the limitations of wind or human and animal muscles. Oil then followed as an even more convenient and efficient fuel than coal, allowing humankind to draw on vast resources of power and so increase dramatically its consumption of energy.In the last few decades we have recognized two very stark and significant facts. First, the supply of these fuels is finite. Secondly, by burning these fossil fuels we are releasing vast quantities of carbon dioxide into the atmosphere, adding significantly to the greenhouse effect and its potentially catastrophic impact on sea levels, weather patterns and agriculture. So, a new revolution is required to provide alternative forms of energy which are both sustainable and kinder to the environment.
In studying future energy requirements it is useful to identify two separate categories. First are static energy needs - heating and cooling of buildings, lighting of buildings and public places, driving stationary machines and appliances in homes and factories and supporting the infrastructure that deals with the provision of water and other utilities. In general these energy demands are met by piping gas and electricity to the customer from centralised resources. Alternatively, it can be delivered as coal and oil to a local storage facility. Major developments are underway to utilise approaches which reduce fossil fuel consumption in this sector including an increase in nuclear power generation, hydro-electricity, wind farms and the harnessing of tidal forces.
The second category of energy requirement, transport, is different as the fuel has to be carried within the vehicle. Oil's high energy content has made it the principal liquid fuel of choice, with transport accounting for over 25% of energy use (and still growing) in economically advanced countries. An attractive alternative to oil is liquid biofuel, derived from plants, which as they grow will absorb as much carbon dioxide as is subsequently released when they are combusted. This means that the net production of carbon dioxide can be lower than that from burning a fossil fuel. However energy and other inputs are still required for the production of the biofuel and these lead to significant greenhouse gas emissions. The reduction of these energy and other inputs provides real challenges for chemists and engineers, and in collaboration with oil and auto companies, the chemical industry is devoting large resources to the research and development of renewable fuels.Biofuels
Producing liquid biofuels
This unit is devoted principally to the manufacture and use of bioethanol, biokerosine and biodiesel. Hydrogen, which will become more important as a fuel in the future, is discussed in another unit.
Bioethanol
The main route to ethanol is via biological fermentation of sugars. The conversion of carbohydrates into an aqueous solution of ethanol is one of the oldest chemical processes and is, of course, the basis of all alcoholic drinks. Prior to the development of the petrochemical industry in the 1940s and 1950s, this process was also the basis of the industrial manufacture of ethanol, which in turn was used as the feedstock for a very wide range of chemicals, including ethanal (acetaldehyde), ethanoic acid (acetic acid) and thence fibres, plastics and explosives.

Figure 1 A Ford T car: Henry Ford envisaged using ethanol as a fuel for his
early cars. Indeed it was added to certain brands of petrol from the earliest
days of the development of the internal combustion engine as a means of
enhancing an engine's power.
By kind permission of Audrey Warren.
Ethanol is made in the same way for fuels as for beverages. The core of the process is fermentation, which is the breakdown of simple carbohydrates (maltose, sucrose, glucose) from biomass to form ethanol. The carbohydrates used to make bioethanol vary according to geography, climate and tradition. For example, sucrose (sugar) is the basis of the process in Brazil (from sugar cane) and in Europe (from sugar beet). Starch from wheat and maize (corn) is preferred in the US and these cereals are also used in Europe.
Production of bioethanol
All recently built bioethanol plants begin the process of making bioethanol by grinding the entire corn kernel or other grain that contains the starch flour. The process is known as dry milling and the flour as meal.There are various methods for releasing the starch from the meal. In one, the meal is mixed with water to form a slurry (the mash) and heated to ca 400 K under pressure, a process that releases the starch molecules. Alternatively, a mixture of enzymes (a-amylases and glucoamylases) is added to the mash at a lower temperature.
The mash is then transferred to large tanks (fermenters) at 360 K and an enzyme (a-amylase) is added, which breaks down the starch into simpler carbohydrates, for example:
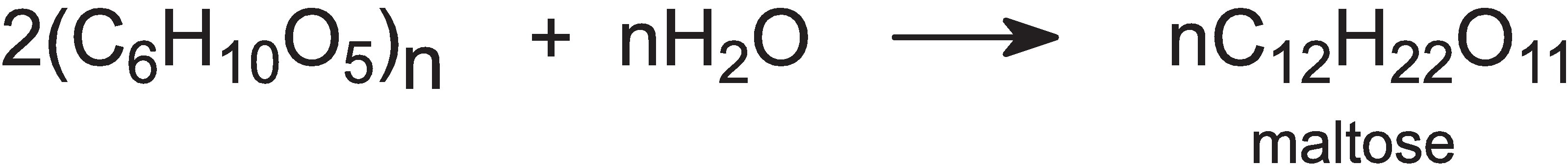
The mash is cooled to 350 K and the enzyme, a-amyloglucosidase, is added and the carbohydrate is hydrolyzed to glucose:
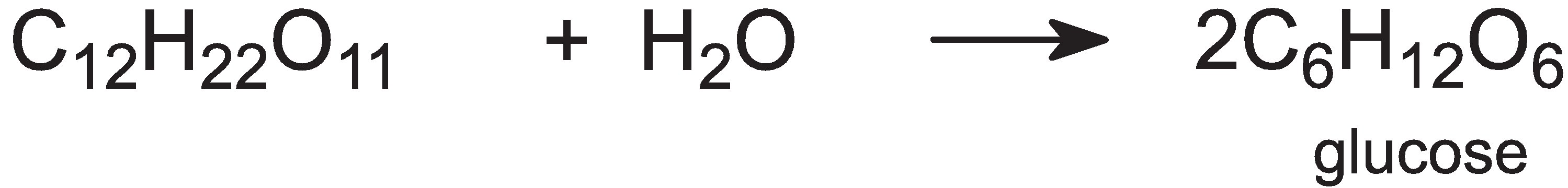
The mixture is cooled further, to 310 K, and live yeast is added, which ferments the glucose into a 'beer', an aqueous solution of ethanol, and carbon dioxide:
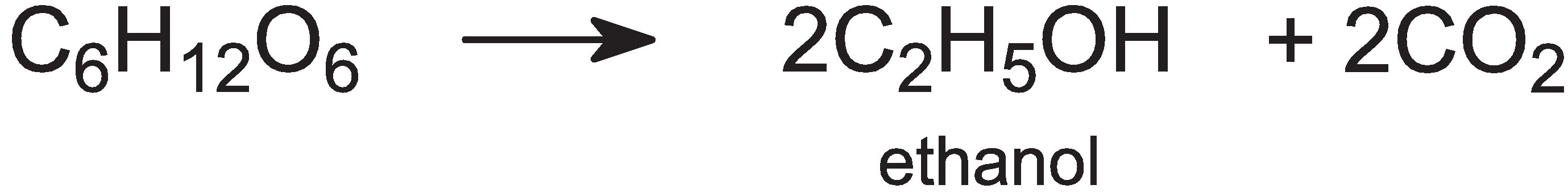
This process takes about 2-3 days, during which the mash is stirred and kept at ca 310 K. The maximum concentration of ethanol produced is ca 8-12% (w/v); higher concentrations kill the yeast.
The solid residue from the fermentation is used as a high protein animal feed.
On distillation, the concentration of ethanol is increased to 96%. However at this concentration, an azeotrope (constant boiling mixture) is produced, which means that it cannot be further concentrated by simple distillation. With further boiling the 96% mixture distils over as if it was a single compound. To obtain pure ethanol, this liquid is passed through a bed of molecular sieves, which retain the molecules of water.
| Figure 2 A new plant to produce bioethanol at Wilton, in the northeast of England. 1 Receiving grain 2 Grain milling 3 Animal feed drying 4 Fermentation 5 Distillation of dilute solution of ethanol 6 Vessels containing a molecular sieve to produce pure ethanol 7 Storage of ethanol 8 Storage of animal feed 9 Control room By kind permission of Ensus. |
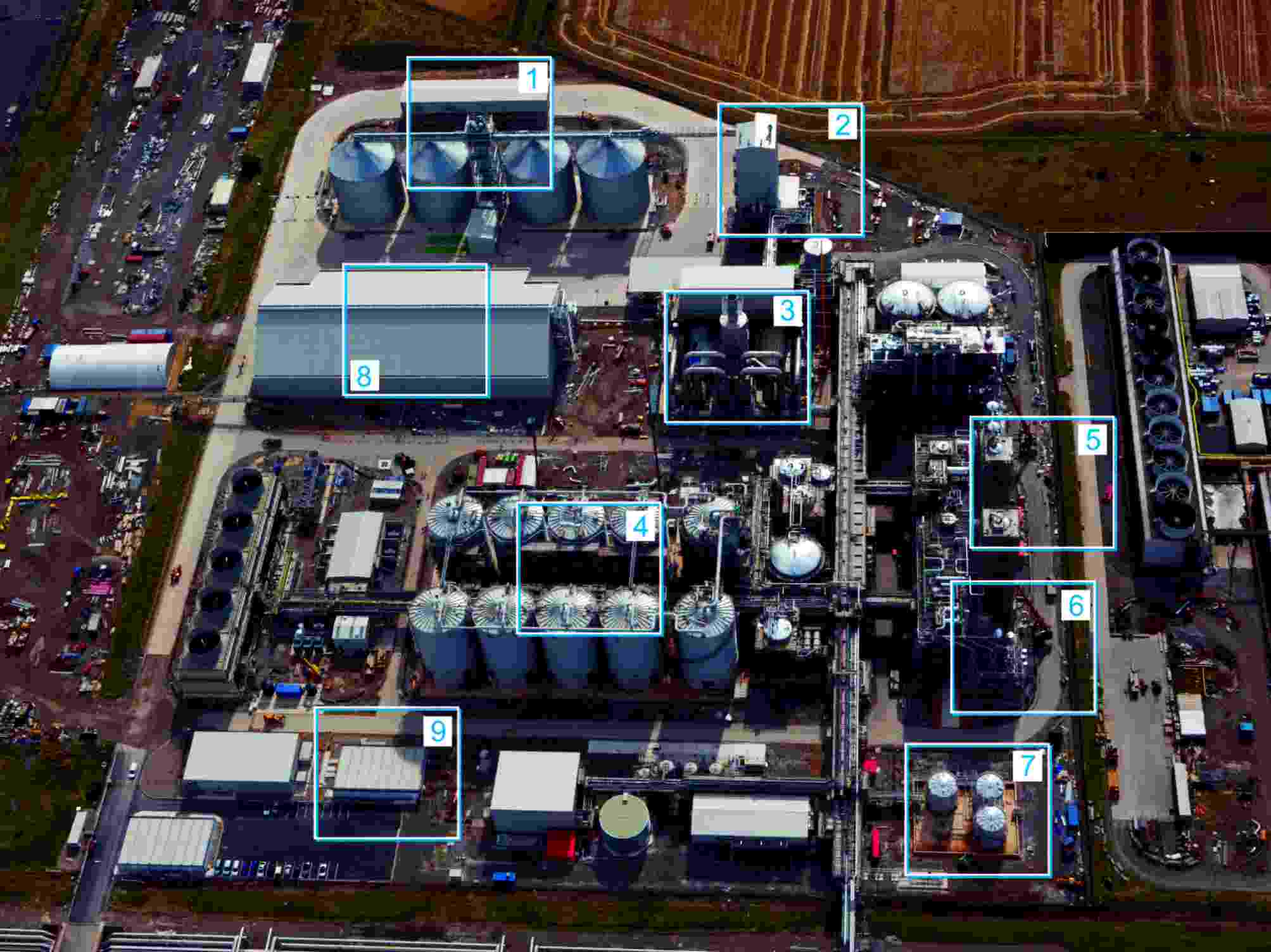 |
New sources of bioethanol
Bioethanol is currently produced using sugars and starch from food crops. There is a great deal of research into other sources of biomass that can be turned into ethanol, including straw, waste wood and non-food crops like grasses (for example, miscanthus (Figure 3)), which would reduce our dependence on using valuable food resources. The barrier is that the polysaccharides in plant material are in the form of lignocellulose, an extremely stable three-dimensional network of lignin and cellulose. Cellulose is a linear polymer of glucose, and lignin is a complex polymer of phenols. Lignocelluloses give plants their rigidity and protect them from herbivores and pathogens. The great stability of lignocelluloses means that to make the carbohydrate available for fermentation requires costly mechanical and chemical pre-treatment such as acids, alkalis or high temperatures. Thus great effort is being made to find enzymes to replace these chemical methods.

Figure 3 Miscanthus growing in Urbana, Illinois, US.
The post in the picture is ca 3 m tall.
By kind permission of Dr Emily Heaton.
Pre-treatment releases the lignin and cellulose. The lignin is burned to provide heat and power for the process. The cellulose, unlike starch, cannot be hydrolyzed to sugars using amylase enzymes so it requires either further acid treatment or sophisticated cellulase enzymes to produce fermentable sugars. However, once the sugars are produced, they are fermented in the same way as described above.
Research is also being conducted on converting biomass to ethanol via synthesis gas (carbon monoxide and hydrogen). The first part of the process is the production of the syngas by heating the biomass at high temperature (1600-1800K) with oxygen or steam. Two methods to produce ethanol from syngas hold out promise. One is by chemical means, passing the gas over a heated catalyst, based on nanoparticles of rhodium. The other is by biological conversion of the gas, feeding it to anaerobic bacteria such as Clostridium ljungdahlii which ferment it to ethanol
Limitations of ethanol as a fuel
Ethanol is hygroscopic (absorbs moisture from the air) and thus is difficult to store and cannot be transported through existing fuel pipelines. It also has a lower energy density than petrol (Table 1).
| Fuels | Energy Density/MJl-1 |
|---|---|
| petrol | 35.5 |
| biodiesel | 33.9 |
| ethanol | 21.6 |
Table 1 Energy density of some liquid fuels.
Another problem with using pure bioethanol is that its vapour pressure on cold days is low and starting the engine can be difficult. Conversely on hot days, its vapour pressure is too high and vapour escapes into the atmosphere from the fuel tank. Producers of petrol get round these problems by using a blend of petrol and ethanol, most commonly 5% ethanol
Uses of bioethanol
Although most bioethanol is used as a fuel, either mixed with petrol (which saves altering the engine) or as pure ethanol (for which the engine needs to be modified), it is expected that bioethanol will become a major source of chemicals. The reactors needed for this use of ethanol are similar to those used in an oil refinery but as the source is biomass, the plant is known as a biorefinery. Bioethanol is being used to make ethene and hence poly(ethene). It is also being used to make ETBE, ethyl t-butyl ether, an important additive to enhance the octane rating of petrol.
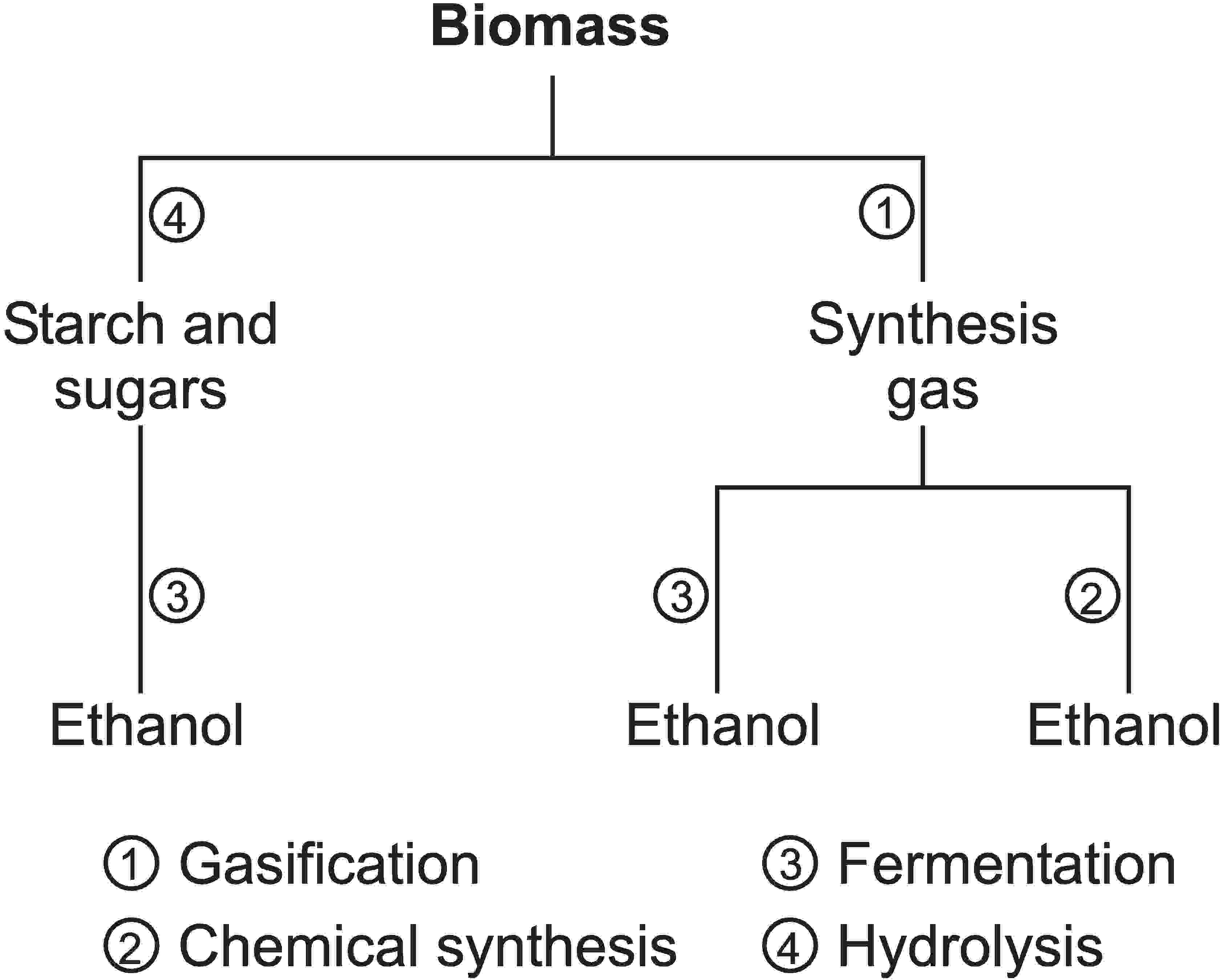
Figure 4 Methods used to produce ethanol from biomass,
which includes gasification, chemical synthesis,
fermentation and hydrolysis.
Biokerosine and biodiesel
Kerosine and diesel fuel are obtained from the distillation of oil and both are made up principally of straight chain alkanes. Kerosine is a lower boiling fraction and is used as a jet fuel. The alkanes contain 10-16 carbon atoms. Diesel fuel used in car and lorry engines is a higher boiling fraction and its alkanes contain 14-20 carbon atoms.
Much work has gone into producing fuels from renewable resources that can be used instead of these petrofuels (fuels derived from petroleum) and they are already being used in mixtures with both petroleum-derived kerosine and diesel fuel.
Manufacture from vegetable oils
Vegetable oils can be converted to biokerosine and biodiesel by the fatty acid methyl ester (FAME) process. Oils, for example from rapeseed, soya and jatropha, are esters of propane-1,2,3-triol (glycerol) and long chain carboxylic (fatty) acids, containing 10-22 carbon atoms.
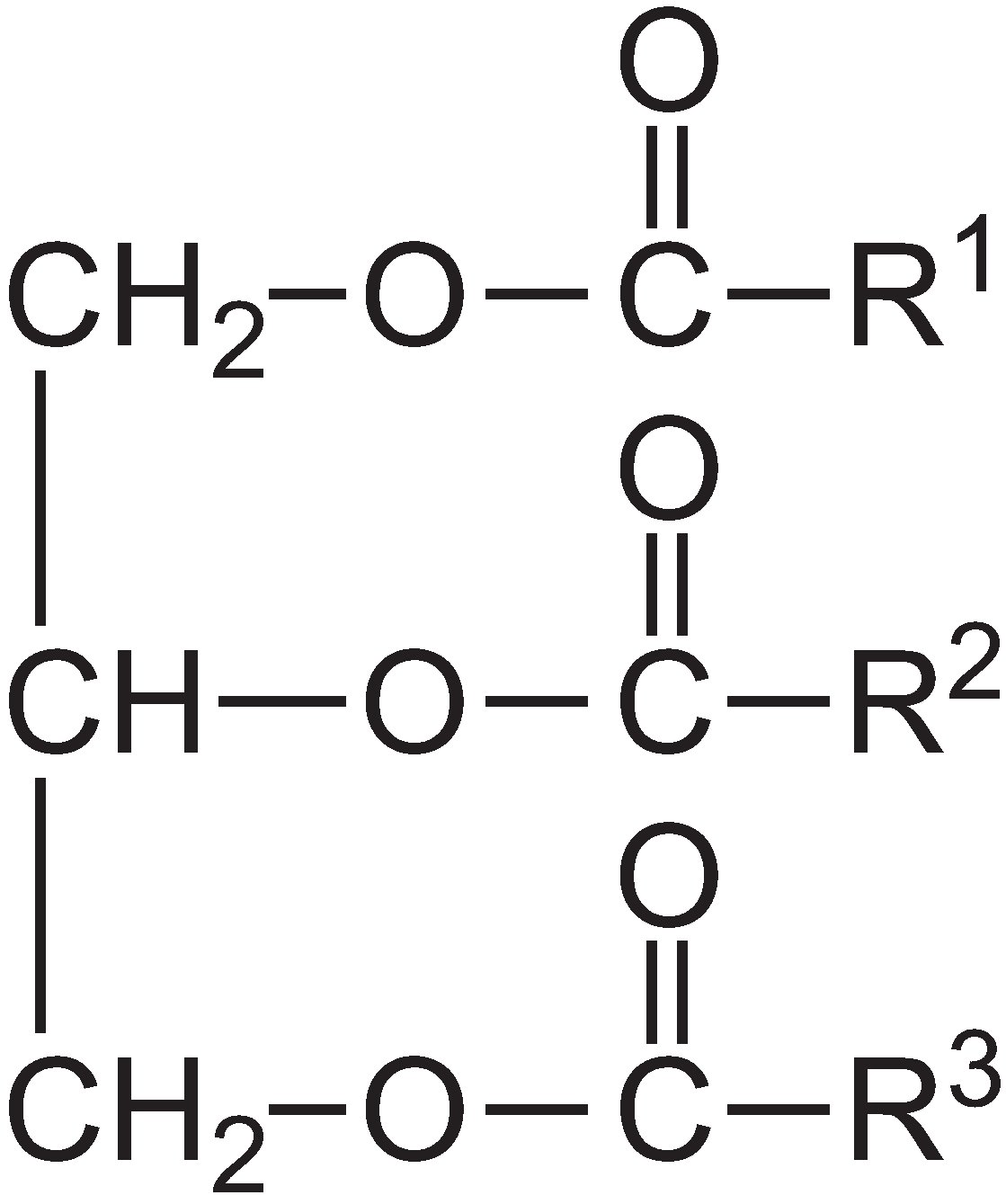

Figure 5 A jatropha plant.
By kind permission of Air New Zealand.
Both virgin oils and waste cooking oils can be used to make these biofuels. The vegetable oil is heated with an alcohol (typically methanol is used) and an aqueous solution of an alkali (potassium hydroxide or sodium hydroxide) added as a catalyst. The glycerol molecule from the vegetable oil is exchanged with methanol to form another ester:
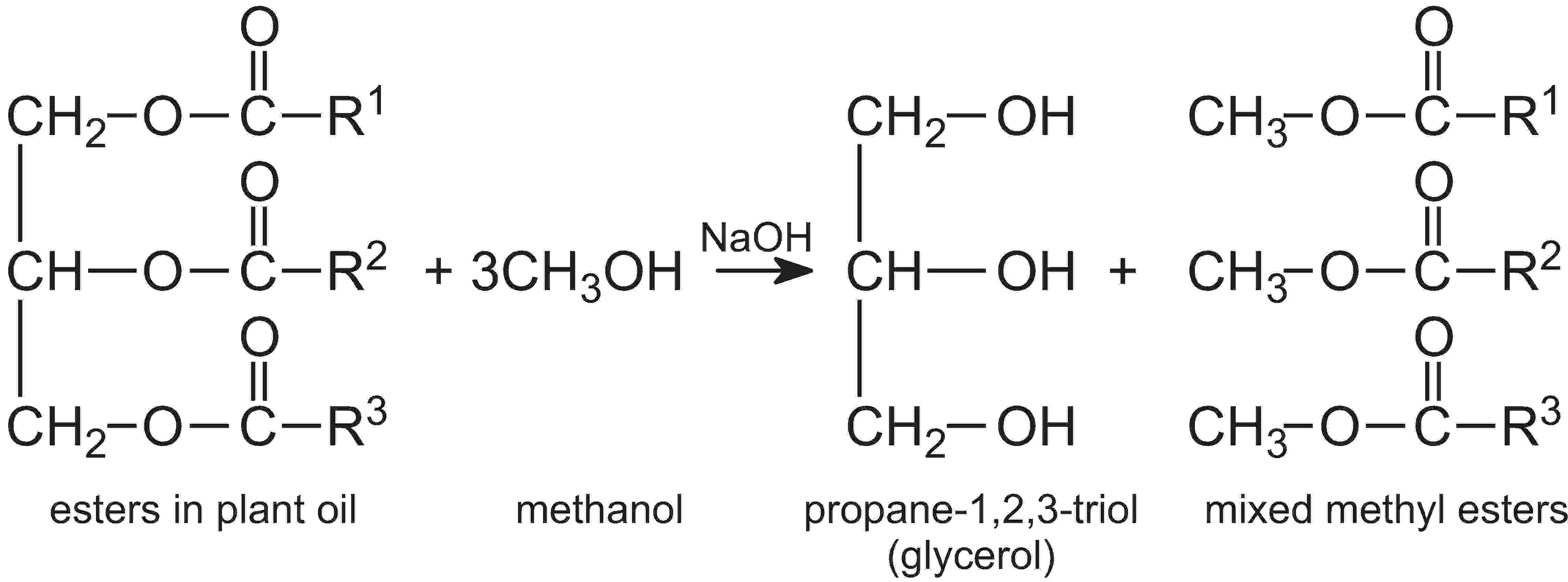
This is an example of a transesterification process.
The mixture of methyl esters is used as a fuel but it is viscous at low temperatures. However, when the transesterification is carried out with ethanol (bioethanol), the ethyl esters do not suffer this problem.
Further, esters produced from saturated fatty acids, which have no double bonds, have a higher freezing point than those from unsaturated acids, which contain one or more double bonds. Thus, oils such as rapeseed which contains esters of poly-unsaturated acids and yield unsaturated methyl (or ethyl) esters which are better for use in cold climates than corresponding esters derived from the more highly saturated palm oil.

Figure 6 Two F/A-18 Hornet strike fighters from the U.S. Navy flight demonstration squadron,
the Blue Angels, are performing tight formation manoeuvres during an air show, part of a
commemoration of the centennial anniversary of naval aviation. The photo shows the first
time the Blue Angels performed with a 50-50 blend of biokerosine and kerosine (jet fuel).
By kind permission of the U.S. Navy (Wikimedia Commons).
Glycerol is a very useful co-product of transesterification. Once the contamination by ethanol (or methanol) and spent catalyst is removed, it can be used in the preparation of cosmetics, toiletries and pharmaceuticals. It is also used to make alkyd resins for paints.
An alternative source of biofuels from vegetable oils involves hydrogenation of the oils over a platinum or nickel catalyst. The product is a mixture of straight chain hydrocarbons which is superior to biodiesel made up of mixed esters produced from vegetable oils because it has no storage stability problems for it is not subject to microbial attack and it has excellent performance in cold climates compared to the esters produced by the FAME process.
Manufacture from biomass
Besides the process described above, much other research is being carried out to produce C10-C20 hydrocarbons from biomass rather than from vegetable and other oils.
Two ways are being introduced to obtain hydrocarbons from biomass which are similar to those obtained from crude oil for use as petrol, kerosine and diesel fuel.
(i)Thermochemical conversion of biomass
Thermochemical conversion uses gasification to convert biomass to synthesis gas (syngas - a mixture of carbon monoxide and hydrogen) at a consistent, designed ratio with minimal additional components and contaminants such as sulphur, tars and solids. Gasification is a very flexible process so, in principle, any biomass including waste can be used to produce the syngas. It is carried out at high temperature (1600-1800K) in a stream of oxygen or steam. This resulting synthesis can then converted into useable fuels and chemicals, for example, hydrogen, methanol and ethanol.
Syngas can also be converted to a mixture of alkanes by the Fischer-Tropsch process (FT). The basic reaction can be represented by:

Although this type of reaction was being used over 100 years ago, much work has been done recently by chemists and engineers to make it a much more efficient. Recent developments of the process include the Shell Middle Distillate Process (SMDS) used in Malaysia and the new SASOL process, developed originally in South Africa. The FT reaction uses iron or cobalt catalysts and is performed at an operating pressure of 20-40 atm and a temperature range of either 470-520 K or 570-630 K.

Figure 7 Part of the Shell Middle Distillate Process plant at Bintulu, Sarawak, Malaysia.
By kind permission of Shell International Ltd.
The products at the lower range are waxy which can be hydrocracked to hydrocarbons which can be used in liquid fuels, kerosine and diesel fuel, for example:

The higher temperature range produces more volatile hydrocarbons, for petrol. The FT process can also be tuned to produce other chemicals, lubricants and hydrogen.
The fuels produced by this method are superior to those produced by the FAME process described above.
(ii) Hydrolysis and fermentation of biomass
Another promising route is based on producing 2-methylpropanol (isobutanol) from biomass, first hydrolysing the cellulose to sugars (including glucose and two of its isomers, mannose and galactose) and then fermentation with specific enzymes from yeast, an example of a BtL (Biomass to Liquid) process.

Figure 8 The Gevo Hydrocarbon Demonstration Facility, Silsbee, Texas, USA, is
producing, from biomass, 2-methyl-2-propanol (isobutanol) which is then
converted to 2-methylpropene (isobutene)and hence biofuels.
By kind permission of Gevo, Inc.
The process can be optimized to produce high yields of 2-methyl-2-propanol.On dehydration, the alcohol yields 2-methylpropene (isobutene):
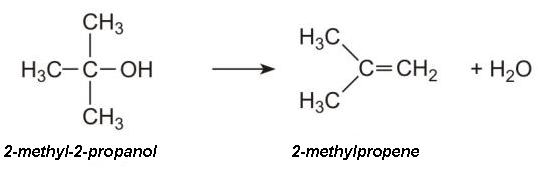
It is this alkene which can be used to produce a wide range of hydrocarbons for use as fuels. For example, 2-methylpropene is readily dimerized over an acid catalyst (hydrogen fluoride or a zeolite) to 2,4,4-trimethyl-2-pentene (iso-octene):
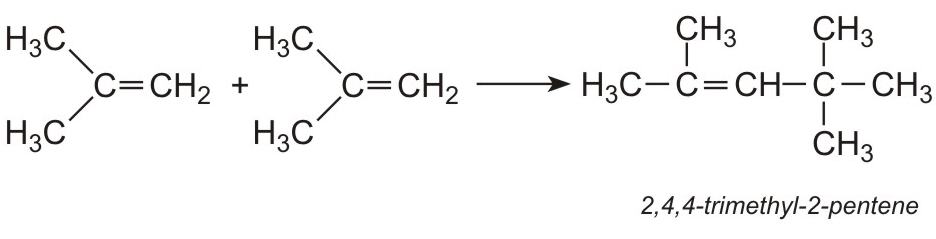
The alkene is hydrogenated, using nickel as the catalyst, to form 2,2,4-trimethylpentane (iso-octane), used to improve the octane rating of petrol:
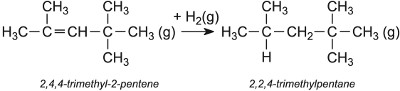
If uncontrolled, there is further reaction between 2-methylpentene and 2,4,4-trimethyl-2-pentene leading to C12 (for example 2,2,4,6,6-pentamethyl-3-heptene) and higher alkenes, which on hydrogenation give a mixture of C12 and higher alkanes.
At first sight, it looks as if these could be used as fuels in jet and diesel engines but the engines perform better using straight chain hydrocarbons which have a higher cetane rating.
However, if a zeolite modified with a trimethylpyridine is used which is shape-selective, a much higher proportion of hydrocarbons with little branching, are formed which make high quality diesel fuel. The principles of shape-selective catalysts are discussed in the unit on catalysis.
Another route to produce kerosine (jet fuel) is being explored, a Friedel-Crafts reaction reacting benzene with 2-methylpropene to form (1,1-dimethylethyl)benzene (t-butylbenzene).
These biofuels have been trialled successfully in jet and diesel engines, both as mixtures with petrofuels and by themselves.
Biobutanol
Butanol can substitute for both petrol and diesel. The most promising method at present for producing biobutanol appears to be by fermenting biomass using the bacterium, Clostridium acetobutylicum. The method is known as the ABE process (it produces acetone (propanone), butanol and ethanol). The feedstocks used successfully so far include sugar beet, sugar cane, wheat, sorghum and cassava. The products are then fractionally distilled, to produce pure butanol.
Bio-oil
The biomass can also be pyrolysed. There are many studies attempting to find conditions in which high yields of useful chemicals are produced. The conditions are very important and must be carefully chosen to prevent carbon (char) being the major product (although it could be used as useful fuel). One way that is being tried is to use very short reaction times, a process known as fast pyrolysis, at about 750-800 K. Some studies have shown that if the biomass is heated gently (ca 500 K) beforehand (a process known as torrefication), the yields of volatile products is increased.
The liquid that is produced, bio-oil, is a mixture of oxygenated compounds including alcohols, aldehydes, ketones and phenols. It can be burnt to produce heat but would become more valuable if it could be made into a liquid fuel (for gasoline and diesel engines) or as a source of chemical feedstocks.
Bio-oil can be reduced catalytically to hydrocarbons. One promising route is to reduce the mixture with hydrogen over a variety of catalysts (examples are molybdenum with either nickel or cobalt on a support of alumina). The mixture of hydrocarbons can be cracked, in a similar way to the cracking of gas oil, to yield a gas, containing alkanes and alkenes and a naphtha-like liquid which can be steam cracked to yield ethene, propene and buta-1,3-diene These are all major feedstocks for a variety of important chemicals.
Alternatively, other routes can lead to a high proportion of aromatic hydrocarbons (benzene and the methylbenzenes (toluene and the xylenes) which could be used in gasoline, to improve its octane rating or as a chemical feedstock.
The pyrolysis of biomass feedstocks can be performed at the biorefinery itself or in decentralised units closer to the biomass production/harvesting site. By doing this close to the harvesting, problems in transporting low grade biomass material, which is very bulky, high in water content, unstable (biological breakdown) and difficult to transport efficiently, are reduced by transporting bio-oil, which is much denser and with significantly reduced water content.
Synthetic Natural Gas (SNG)
At first sight, the words natural and synthetic within the term synthetic natural gas seem to be a paradox. SNG is in fact methane produced synthetically from biomass. Anaerobic digestion is the treatment of non-woody biomass by micro-organisms in the absence of oxygen. The products of digestion are biogas, a mixture of methane and carbon dioxide, and digestate, a nutrient-rich waste. The biogas can be combusted to generate heat and power or further processed into a liquid fuel. The digestate can be used to condition soil.
Some concerns about biofuels
Three driving forces for the production of biofuels are:
- saving a valuable finite resource - fossil fuels which will become more expensive as they become scarcer
- climate change – the need to reduce the emissions of carbon dioxide
- energy security - countries with a shortage of crude oil and natural gas believe that they are vulnerable and want to develop their own fuel supplies.
However, there are criticisms of the large scale production of all biofuels. First, food is becoming scarcer as plants to produce biofuels are grown on land which otherwise could be used for food production. There will be a consequent increase in the price of food affecting particularly the poor in developing countries. Although this criticism is usually aimed at the production of bioethanol, it also applies to the production of palm oils for biodiesel and other biofuels.
Secondly, energy is used to produce the crops (in the manufacture of the fertilizer, in the sowing, looking after and harvesting the crops and their transport to the refineries). Energy is also used in the fermentation processes and in the subsequent purification of the fuels. Some of the methods for producing biofuels are actually thought to cause great harm to the environment. Thus, much work is being devoted to ensure that the energy inputs are reduced substantially and that the carbon dioxide produced is captured. One promising development is the production of bioethanol from wheat, being pursued in the UK and six other European countries. Besides the bioethanol, a particularly nutritious animal feed is produced (a wheat protein concentrate) (Figure 2) and the carbon dioxide is captured and used in the food and agricultural industries. The energy not needed for the process is then fed into the electricity grid. However, even if all the manufacturing processes are optimised using today's technology and knowledge, biofuels from agricultural crops can only replace a relatively small proportion of transport fuels and therefore do not currently offer the most significant solution to the great environmental challenges facing the world.
Date last amended: 5th April 2014
