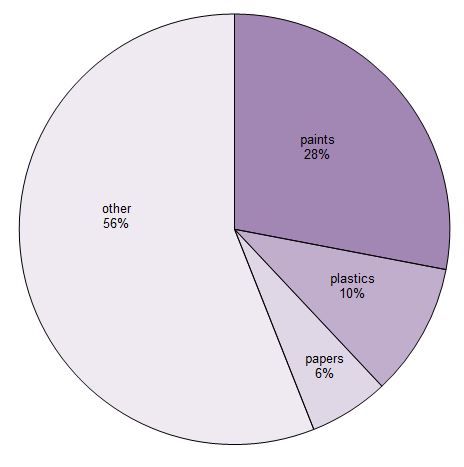
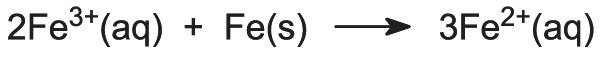Uses of titanium dioxide
Titanium dioxide is the most used white pigment and provides whiteness and opacity for paints and coatings (including glazes), plastics and paper.
It has many speciality uses. It is resistant to UV radiation and thus does not discolour over a long period of time and ultra pure and fine crystal titanium dioxide grades are being increasingly used for sun screens.
It is also being used in novel nanotechnology applications.
Figure 1 Uses of titanium dioxide
Data for 2013, Merchant Research and Consulting, 2015
Much smaller amounts of titanium dioxide are used as a semi-conductor and to catalyse the photodecomposition of water into hydrogen and oxygen.
It also has a high dielectric constant and a high resistance and thus it is used to make capacitors.
The strong bonding between titanium and oxygen gives great thermal stability, melting at 2100 K. It is thus used as a ceramic material. It is also used to increase the acid resistance of vitreous enamels.
Annual production of titanium dioxide
| World | 5.7 million tonnes1 |
| China | 2.7 million tonnes1 |
| US | 1.3 million tonnes2 |
| Europe | 1.3 million tonnes1 |
1. Data for 2013, Merchant Research and Consulting, 2015
2. 2018 Elements of the Business of Chemistry, American Chemistry Council.
Manufacture of titanium dioxide
There are two main processes, the Sulfate Process and the Chloride Process (Table 1) which use the two principal ores, ilmenite and rutile, respectively. Ilmenite contains 45-60% TiO2 and rutile contains up to 99% TiO2. The ores are mined worldwide but most production is in Australia and South Africa.
Each large producer of titanium dioxide balances its production between the two processes. Each produces the oxide in the rutile crystal form but the Sulfate Process can also produce another form of the oxide, anatase, which is softer and which is used for a small number of specialist applications.
The Sulfate Process is run as a batch process; the Chloride Process is run as a continuous process.
It is estimated that about 65% of the world's production is based on the Chloride Process.
| Sulfate Process | Chloride Process |
|---|---|
| long established and simple technology | new technology |
| uses lower grade, cheaper ores | needs high grade ore |
| batch process | continuous process |
| large amounts of waste materials | small amounts of waste formed with toxicity problems: Cl2 and TiCl4 |
| pollution control expensive | recovery and recycling of chlorine possible |
| produces anatase and rutile pigments | only produces rutile pigments |
Table 1 Comparison of the two processes for the manufacture of titanium dioxide.
The Sulfate Process
The chemistry of the process involves three main stages:
a) dissolving the ore
b) formation of hydrated titanium dioxide
c) formation of anhydrous titanium dioxide
(a) Dissolving the ore
The ore is usually ilmenite, FeTiO3. It is ground finely and dissolved in sulfuric acid to form a mixture of sulfates:
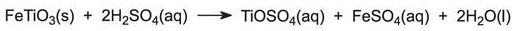
Before the titanium dioxide is extracted, the iron ions must be removed from the solution so that the colour of the final product is not spoiled. The solution is therefore reacted with recycled iron sources to convert any iron(lll) ions that may be present to iron(ll) ions:
The solution is allowed to stand so that the unreacted solid settles, and the clear liquid is poured off before being concentrated by evaporation. Cooling then allows light green crystals of iron(ll) sulfate to form and to be filtered off. These are sold separately. The remaining solution contains titanyl sulfate, TiOSO4.
(b) Formation of hydrated titanium dioxide
The next stage involves the hydrolysis of the titanyl sulfate in solution to give insoluble, hydrated titanium dioxide:
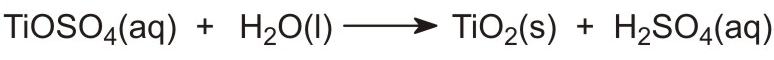
This is a critical stage and the conditions must be controlled to ensure that the precipitate is suitable for filtering and roasting.
(c) Formation of anhydrous titanium dioxide
The final stage of the process is the heating of the solid in a furnace, known as a calciner. This is a rotating cylinder which is typically heated by gas flames. As the cylinder turns, the titanium dioxide passes along it and its temperature rises from 313 K, as it enters, to over 1000 K as it leaves:
Heating evaporates the water and decomposes any remaining sulfuric acid in the solid. After cooling, the product is 'milled' to form crystals of the size needed. Crystals may also be coated with another substance, such as aluminium oxide or silica, to make the titanium dioxide mix more easily with liquids or to make the water-based paints made from it last longer.
This coating is achieved by dispersing the dry product from the calciner in water containing the dissolved coating chemicals which precipitate from solution onto the TiO2 crystals. The coating is usually between 3 and 8% by weight in the final dried pigment. This coating is achieved by changing the temperature and pH of the solution. Each TiO2 crystal needs to be coated uniformly to maximize the effectiveness of the coating. The coated TiO2 crystals are filtered from the water and dried before being packed for dispatch to the final customer.
The Chloride Process
There are two main stages:
a) the conversion of rutile to titanium(IV) chloride
b) the oxidation of titanium(IV) chloride
(a) The conversion of rutile to titanium(IV) chloride
The rutile is fed into a heated bed together with a source of carbon, usually coke. Chlorine is fed into the bed and the reaction takes place to form titanium(IV) chloride in the vapour form which is removed from the bed. Iron and other metals in the ore are chlorinated and also leave the bed in the vapour state. The oxygen in the ores is combined with the carbon to form carbon monoxide and dioxide. The vapour stream is cooled and the metal chlorides other than titanium(IV) chloride are condensed and solidified. The titanium(IV) chloride vapour, which contains almost pure titanium(IV) chloride and has a lower boiling point, is then condensed and stored as liquid. It is then reboiled and distilled to give a purer product to feed to the next stage.
(b) The oxidation of titanium(IV) chloride
Liquid titanium(IV) chloride is vaporised and burnt in oxygen, together with a hydrocarbon fuel source (for example, methane) to a high temperature to initiate the reaction and keep the temperature high enough for the reaction to proceed:
The titanium dioxide is formed (by adding seed crystals) as a fine solid in the gas stream and is filtered out of the waste gases using cyclones or filters. Once again control of crystal growth is important to give particles of the correct size for pigments. This is done by adding nucleating agents to the gas stream (e.g. water or aluminium chloride) and by cooling the products. The chlorine in titanium(IV) chloride is released and recycled to the chlorination stage of the process above.
The product contains small amounts of absorbed chlorine gas which are removed. The product is washed and dried before milling and surface treatment in an identical manner to that used in the Sulfate Process described.
Date last amended: 27th November 2018