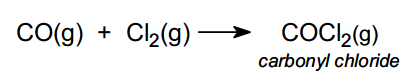
Uses of polycarbonates
There are many polycarbonates which vary in properties depending on their molecular mass and structure. As the molecular mass increases, the polymer becomes more rigid. Further, the properties are changed by blending it with other polymers, for example, with ABS and polyesters such as PET.
Because of their remarkable properties (they are flame and heat resistant, tough and transparent), the polymers are very widely used.
|
Among the uses of the polycarbonates and their blends are:
- medical (for example, for dialysis housing and spectacle lenses)
- electro-electronic (for example, sockets, lamp covers,
- fuse-boxes, computer and television housings)
- construction (for example, stadium roofs, signs, skylights)
- optical storage (CDs, DVD, HD-DVDs)
- cars (interior lighting and headlamps, sunroofs, side windows, radiators, grilles, bumpers)
- packaging (for example, large water bottles)
|
Annual production of polycarbonates
| World | 4.4 million tonnes1 |
| Asia | 2.0 million tonnes2 |
| Europe | 1.5 million tonnes2 |
| US | 0.9 million tonnes2 |
1. 2016 estimate Merchant and Research Consulting, 2014
2. 2016 estimate from reference 1 and PlasticsEurope, 2015
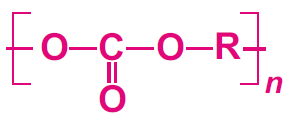
Manufacture of polycarbonates
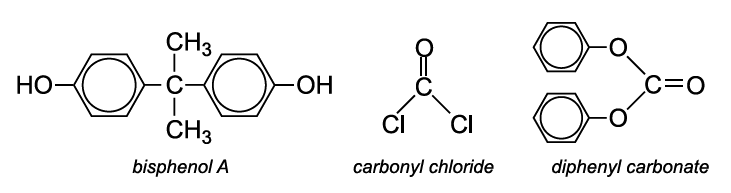
The polycarbonate most used is manufactured by condensation polymerization between bisphenol A and either carbonyl chloride or diphenyl carbonate.
Bisphenol A is produced by the condensation of phenol with propanone.
Carbonyl chloride is produced from carbon monoxide and chlorine:
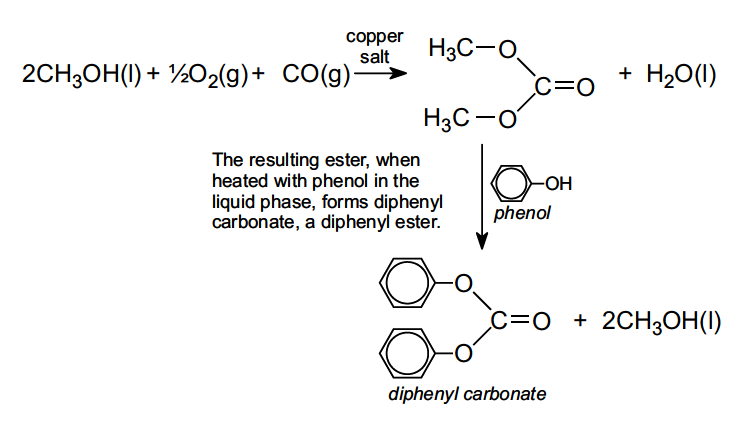
Diphenyl carbonate is produced from dimethyl carbonate, which is often made from methanol, oxygen and carbon monoxide in the liquid phase in the presence of a copper salt such as copper(II) chloride:
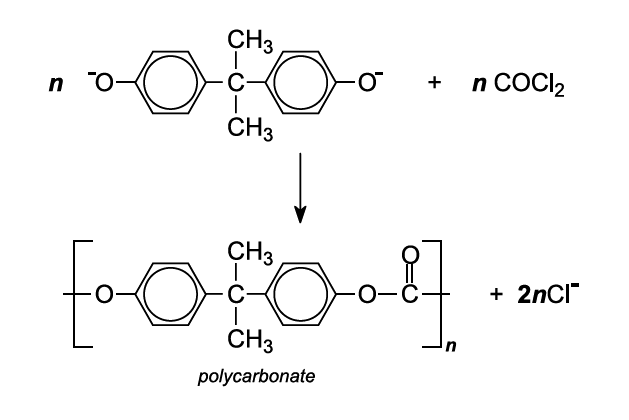
The polymer is usually formed by the reaction of bisphenol A and carbonyl chloride in a basic solution.
A solution of bisphenol A in sodium hydroxide (i.e. a solution of the sodium salt of the phenol) is prepared. It is mixed with a solution of carbonyl chloride in an organic solvent (dichloromethane). The polymerization takes place at the interface between the aqueous and organic layers with the help of a catalyst (an amine):
The polycarbonate is held in solution in the organic layer. This solution is then run off from the aqueous layer and is either evaporated to form granules of the polymer or ethanol is added to precipitate the solid polymer.
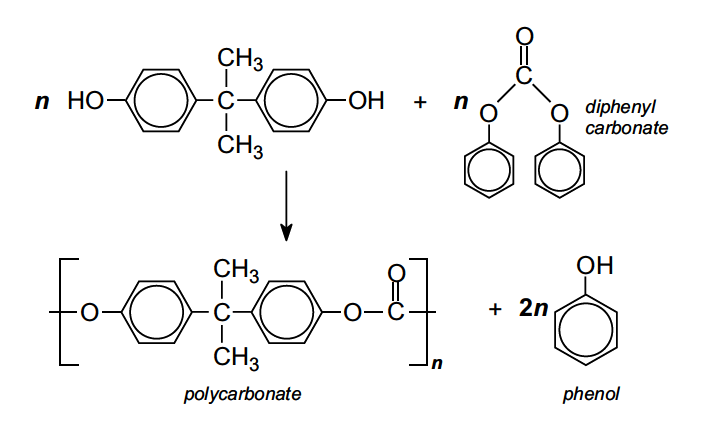
However, an increasing proportion of polycarbonates is made via diphenyl carbonate, in order to eliminate the use of carbonyl chloride, an extremely poisonous gas.
Bisphenol A and the ester are heated together to form a molten mass of polymer:
The phenol and excess reactants are removed by distillation under reduced pressure. The polycarbonate melt is then pressed through fine nozzles to form long 'spaghetti-like' threads, which are cooled down and granulated.
Further developments
Although the polycarbonate derived from bisphenol A is easily the most widely used polycarbonate, co-polymers have been developed in which substituted bisphenols are added and reacted with diphenyl carbonate.
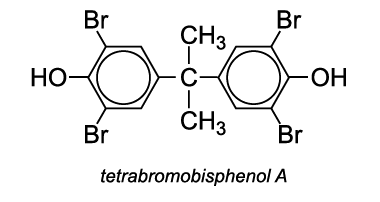
For example, tetrabromobisphenol A is added, prior to polymerization. The resulting polymer has enhanced flame resistance.
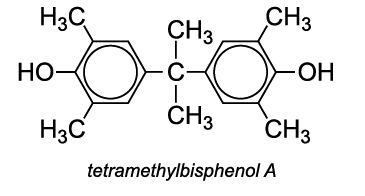
Another co-monomer used is tetramethylbisphenol A, which improves the polycarbonate's resistance to heat.
Date last amended: 27th April 2017