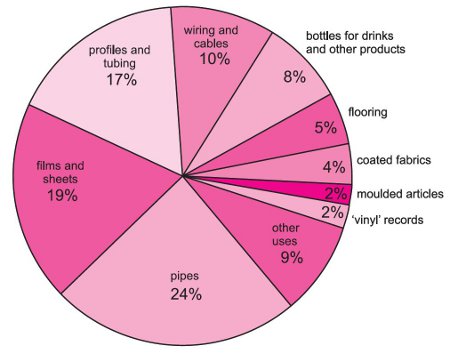
Uses of poly(chloroethene) (polyvinyl chloride)
Poly(chloroethene) is used in building and construction, for example in making window and door profiles and in making pipes (from guttering to sewers, and ducting for cables).
Figure 1 Uses of poly(chloroethene).
It is also much used for packaging, including film for food and bottles. Wire and cable insulation and sheathing, for example in telephones, computers as well as for power transmission cables, is another main outlet for the polymer.
|
Chloroethene (vinyl chloride) is also used to produce co-polymers, for example with ethenyl ethanoate (vinyl acetate). The co-polymer can be processed at much lower temperatures than poly(chloroethene) homopolymers of the same relative molecular mass. It is used for coating metals and wood. The film is flexible, hard wearing, resistant to chemical attack and can be pigmented so that it is particularly suitable, for example, for ships. The co-polymer is also used in adhesives and in inks.
Annual production of poly(chloroethene) (polyvinyl chloride)
| World | 38.5 million tonnes1, 2, 3 |
| Asia | 21.5 million tonnes1 |
| US | 6.9 million tonnes1 |
| Europe | 6.7 million tonnes1 |
| Russia | 0.51 million tonnes4 |
Data from:
1. Data for 2013, Merchant Research and Consulting Ltd, 2014
2. Estimated to be 45 million tonnes in 2017, Merchant Reseaech and Consulting Ltd, 2014
3. Estimated to be 55 million tonnes in 2020, Business Wire, 2011
4. Federal State Statistics Service: Russian Federation 2011
Manufacture of poly(chloroethene) (polyvinyl chloride)
The production of PVC involves several stages:
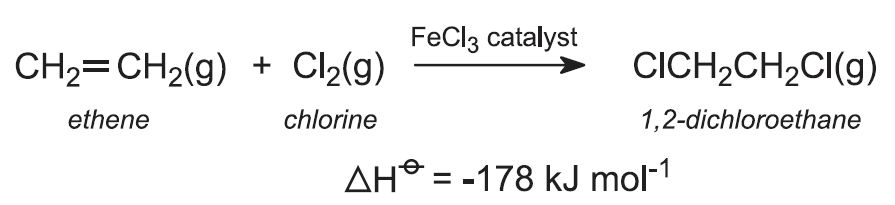
a) ethene is converted into 1,2-dichloroethane
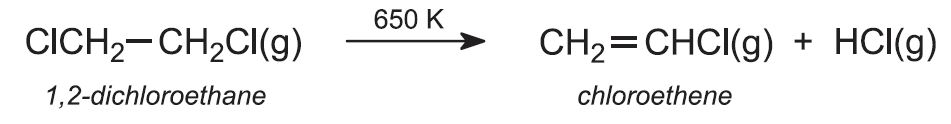
b) 1,2-dichloroethane is cracked to chloroethene (vinyl chloride)
c) polymerization of chloroethene (vinyl chloride)
(a) Production of 1,2-dichloroethane
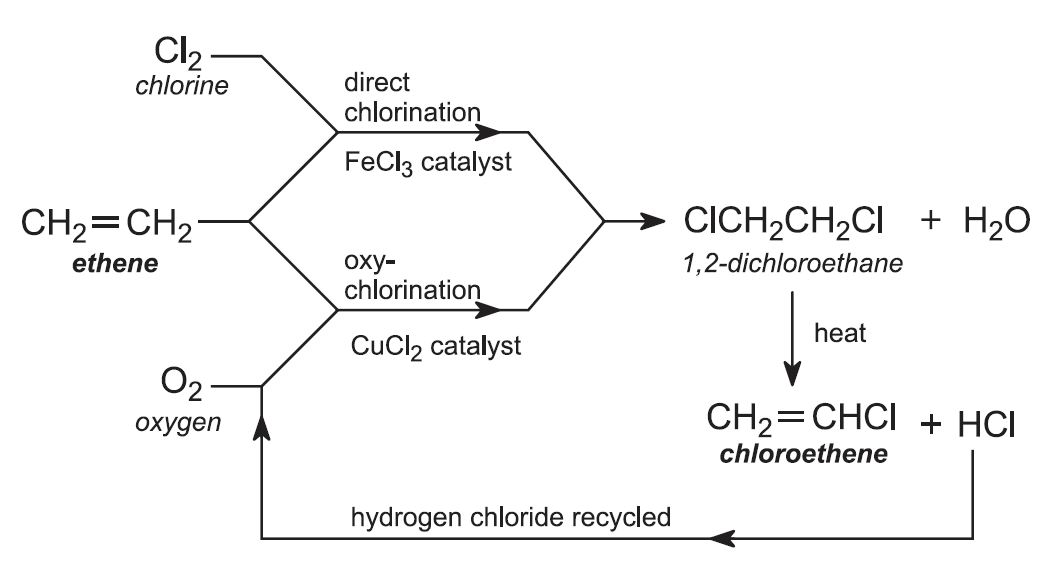
Ethene is obtained from the cracking of ethane, propane, naphtha and gas oil. In practice, two processes for the overall conversion of ethene to chloroethene are used in parallel.
(i) Direct chlorination
Ethene is reacted with chlorine in the liquid phase, using excess chloroethene as solvent. The catalyst, iron(lll) chloride, is soluble in chloroethene. The reaction is exothermic, and no external heat is needed.
The reaction temperature is maintained at 320-350 K and with a pressure of ca 4 atm. Higher temperatures give unwanted polychlorinated compounds.
(ii) Oxychlorination route
The cracking of 1,2-dichloroethane during stage (b) of the overall process forms hydrogen chloride as a by-product. It is unwanted as hydrogen chloride and is very detrimental to the environment. However, it can be used in this second method of making 1,2-dichloroethane.
Ethene is mixed with hydrogen chloride and air (enriched with oxygen). The gases are passed over a heated solid catalyst in metal tubes, in a fluid bed reactor, maintained at ca 500 K and 5 atm.
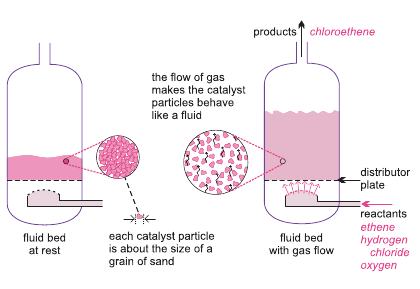 |
| Figure 4 A diagram to illustrate the use of a fluid bed reactor for the oxychlorination of ethene to chloroethene. On the left hand side, the catalyst particles are at rest. On the right hand side, the the particles are now acting as a fluid, as the gaseous reactants pass through the solid. |
The catalyst generally used is a mixture of copper(ll) and potassium chlorides, deposited on alumina.
As the reaction is highly exothermic, the reactor vessel is cooled to give the optimum reaction temperature and to reduce unwanted by-product formation of, for example, chloroethane and 1,1,1,2-tetrachloroethane. 95% conversion of ethene to 1,2-dichloroethane is obtained.
Unreacted hydrogen chloride is removed by scrubbing the gases with aqueous sodium hydroxide solution. Impurities, such as unreacted ethene and unwanted chlorinated hydrocarbons, are removed by distillation. Ethene is recycled.
(b) Production of chloroethene (vinyl chloride)
1,2-dichloroethane is then passed rapidly through metal tubes heated to ca 650 K. It is cracked to eliminate hydrogen chloride:
Thus the above two processes are normally operated in parallel, with the purified 1,2-dichloroethane from both routes cracked to give chloroethene (Figure 5). Chloroethene is separated from hydrogen chloride and unconverted 1,2-dichloroethane in a two-stage distillation process. Hydrogen chloride is removed in the first stage and recycled to the oxychlorination process. The second stage separates chloroethene from unreacted 1,2-dichloroethane, which is recycled to the cracker.
The cracking process has been modified by increasing the volume of the reactor. This increase in volume increases the conversion and reduces the energy required to produce chloroethene.
Figure 5 The manufacture of chloroethene.
(c) Polymerization of chloroethene (vinyl chloride)
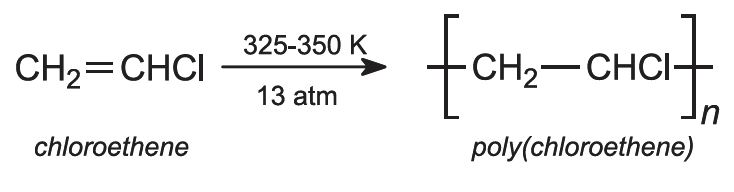
This is an example of addition polymerization. PVC is made by free-radical polymerization in suspension. The monomer (bp 259 K) is polymerized in aqueous dispersion at 325-350 K. Pressure (13 atm) is used to keep the monomer in a liquid phase. For polymerization to be controlled, an initiator is needed.
In suspension polymerization an initiator, an organic peroxide is used, which is soluble in chloroethene. After the reaction, excess monomer is removed and the polymer is separated, by centrifuging and drying.
During polymerization, the polymer precipitates out as it is formed, since it is insoluble in the monomer. It is used for the extrusion, injection moulding and film-making processes.
It is estimated that mearly 80% of the polymer is produced by suspension polymerization.1
1. Business Wire, 2011
Alternatively, chloroethene is polymerized as an emulsion in water. Ammonium peroxodisulfate is often used as the catalyst as it is soluble in water. The monomer is in the form of very fine droplets rather similar to paint. On evaporation after the polymerization, a very fine powder is formed. This is used as a coating on, for example, flooring and wallpaper.
The Future
Ethane is a much cheaper feedstock than ethene. Much work, over many years, has been devoted to developing a process to produce chloroethene directly from ethane, rather than first converting feedstocks, such as ethane to ethene. The first such plant has come on stream in Germany. Ethane, hydrogen chloride and oxygen (separated from air in a nearby unit are heated to ca 750 K over a catalyst. At present only a small plant has been built but if successful large plants will follow.
Date last amended: 29th April 2017