Uses of urea
Urea is the world's most commonly used nitrogen fertilizer and indeed more urea is manufactured by mass than any other organic chemical. Containing 46% N, it is the most concentrated nitrogen fertilizer, and is readily available as free-flowing prills (granules). It is the cheapest form of nitrogen fertilizer to transport and it is also the least likely to 'cake'. It is therefore favoured in developing countries.
 |
Figure 1 This rice paddy field in Thailand has been treated with urea, the nitrogen-containing fertilizer that is most used in developing countries. By kind permission of Kim Dixon. |
While over 90% of urea produced is used as a fertilizer, it has other uses, which include the manufacture of the melamine, used in melamine-methanal resins. Urea itself also forms important resins.
An increasingly important use of urea is in reducing air pollution from diesel engines in cars, buses and lorries. Diesel engines run at high temperatures and nitrogen and oxygen, from the air, are able to react together under these conditions to produce high concentrations of nitric oxide. One way to remove this pollutant is to allow it to react with ammonia to form nitrogen.
However it is not possible to use ammonia directly as it is too volatile and is poisonous. Instead a solution of urea in water is injected into the hot gases emerging from the engine in the exhaust. Urea is thermally decomposed to ammonia and carbon dioxide. This is the reverse of the process used to make ammonia:
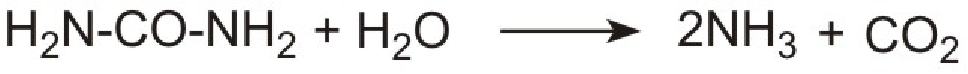
Unlike ammonia, urea is safe and easy to handle.
The products, ammonia and carbon dioxide, together with the exhaust gases, are passed immediately over a catalyst in the exhaust system. Ammonia reduces the oxides of nitrogen (mainly nitric oxide), formed in the combustion processes, to nitrogen. The process is complex but the overall reaction can be represented thus:
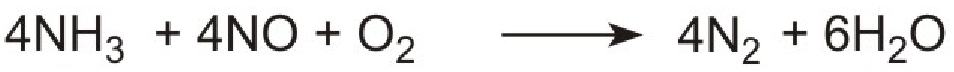
The system is known as Urea SCR (urea-based selective catalytic reduction) and can reduce pollution by nitrogen oxides to almost zero.
| Figure 2 Urea tanks are now standard equipment for most new diesel lorries, buses and cars in many countries. The urea is added to the exhaust gases as a 32% aqueous solution often known as BlueTEC. In this photo, a Mercedes bus is being filled with BlueTEC solution (with the blue nozzle to the lower tank) and diesel (the upper tank). By kind permission of Daimler AG. |
 |
Several catalysts have been used. One series is based on transition metal oxides (for example those of vanadium and tungsten) on a carrier, titanium dioxide. Another series is based on zeolites, in which some of the cations have been exchanged with a metal such copper.
| Figure 3 A Mercedes-Benz E-Class E300 BlueTEC Hybrid car. The diesel engine is combined with an electric motor which allows for purely electric driving even at high speeds which reduces the amount of pollutants significantly compared to a pure diesel engine. The amount of nitrogen noxides emitted is further reduced by the use of BlueTEC solution, which converts the oxides in the exhaust to nitrogen and water vapour. By kind permission of Daimler AG. |
 |
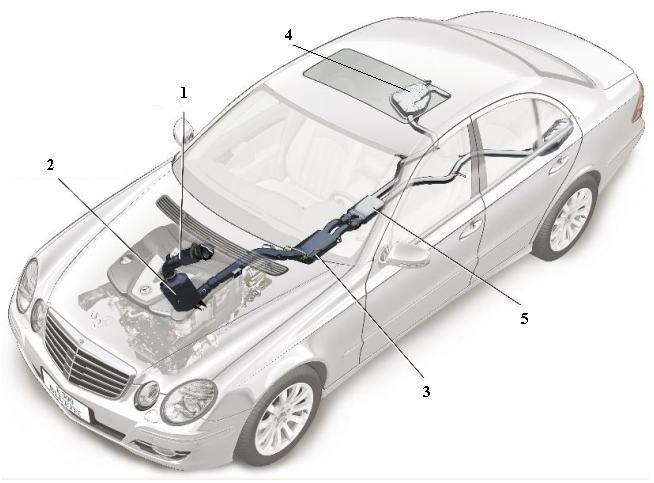 |
Figure 4 A line diagram of the car above illustrating five key elements in the design of the exhaust system. 1 The oxidation catalyst is used to remove unwanted hydrocarbons, ensuring that they are oxidised to carbon dioxide and water. The catalyst is usually based on platinum or palladium. 2 Known as an NOx catalytic convertor, it contains aluminium oxide on whose surface, platinum and barium oxide are present. It traps the oxides of nitrogen. When the solid is saturated with the oxides, unburnt hydrocarbons are allowed to flow through, converting much of the mixture to nitrogen, carbon dioxide and water vapour. 3 A filter which traps particulates (small pieces of carbon and other solids). 4 A tank containing the solution of urea. 5 The SCR-catalytic convertor which contains another catalyst, for example an oxide of vanadium (or tungsten) on titanium dioxide, which allows the exhaust gases, still containing some nitrogen oxides, to react with ammonia formed from the urea solution, to produce exhaust gases with only traces of the oxides. By kind permission of Daimler AG. |
Annual production of urea1
| World | 164 million tonnes |
| China | 62 million tonnes |
| India | 23 million tonnes |
| Middle East | 20 million tonnes |
| Rest of Asia | 18 million tonnes |
| FSU | 12 million tonnes |
| North America | 9.5 million tonnes |
| Europe | 9.5 million tonnes |
It is expected that the global annual production will increase to over 200 million tonnes by 20182.
1. Potash Corporation, 2013
2. International Fertilizer Industry Association, 2014
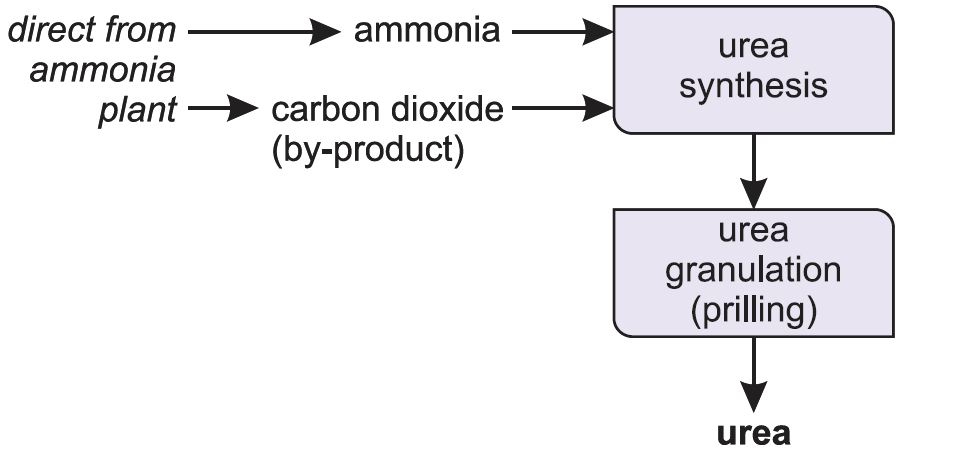
Manufacture of urea
Ammonia reacts with carbon dioxide to produce urea. Urea is always manufactured close to an ammonia plant (Figure 5).
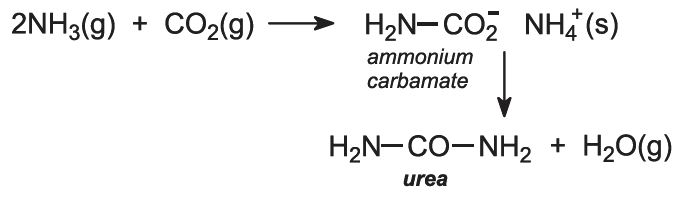
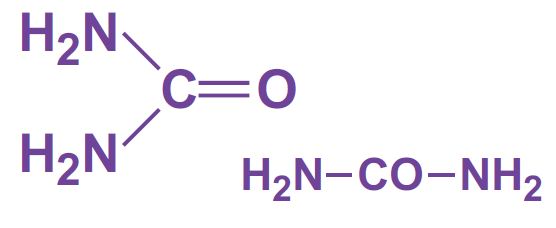
Ammonia and carbon dioxide are heated together at 450 K and 200 atm pressure. First ammonium carbamate is formed, which rapidly decomposes to form urea:

Figure 5 An aerial view of a large plant in Alberta, Canada, in which ammonia is synthesized
and then converted to urea.
By kind permission of Agrium Inc.
Much of the urea is prilled (Figure 6) and sold in that form.
| Figure 6 These small spheres of urea are known as prills. The prills are formed by spraying molten urea down a tower up which air is pumped. They are slightly smaller than urea sold as granules and are particularly useful when the fertilizer is being applied by hand. By kind permission of Agrium Inc. |
 |
Date last amended: 26th January 2017