The feedstock in a conventional refinery is oil which is mostly a mixture of hydrocarbons (including alkanes, cycloalkanes and aromatic hydrocarbons). This is a positive feature because most of our gaseous and liquid fuels are mixtures of hydrocarbons and thus release more energy per gram than for example an alcohol. This is discussed further in the section on biofuels. However, to produce chemicals for the chemical industry, functional groups have to be added. One of very many examples is the conversion of ethane, a saturated compound to the unsaturated ethene, by cracking, and its subsequent oxidation to epoxyethane.
On the other hand, the feedstock in a biorefinery is biomass which is mainly a mixture of polymeric organic compounds which abound with functional groups, mainly containing oxygen (alcohols, aldehydes, ketones, carboxylic acids, for example). This provides a suitable start for making chemicals but the functional groups have to be removed if we wish to maximise the energy output of gaseous and liquid biofuels for engines.
|
Figure 1 Agriculturalists in the European Union are presently investigating the use of agricultural land for non-food production, such as the growth of biomass crops as a source of renewable energy and renewable sources of raw materials. The crop in the photograph is Sorghum (Sorghum bicolor), which is grown extensively in India, China, and Africa where it is the leading cereal. The partners of the European Sorghum Network (Italy, France, Spain, Greece, Portugal and the UK) are studying the production and environmental impact of sorghum under field conditions at various sites around mainly southern Europe. These investigations include studies of soil erosion, crop rotations, and balances of water, nitrogen, carbon and organic matter. By kind permission of Evelyn Simak |
 |
Manufacture of chemicals in a biorefinery
Biomass can be transformed into a wide range of chemicals in two main ways, by:
(a) exploiting the latest developments in biotechnology (particularly fermentation)
(b thermochemical processing routes
A third and emerging route which is of much interest is the conversion of simple carbohydrates obtained from biomass into important intermediates (for example for the plastics industry) at mild temperatures (ca 400 K) in aqueous solution. This is often known as a chemocatalytic process or bioforming and is discussed later in this unit.
These main routes are shown in Figure 2, which represents processes that can be used in a biorefinery and provides the structure for the discussion in this unit. The important chemicals now being produced from biomass are discussed, including ethane and propane, ethene and propene and the polymers poly(ethene) and poly(propene), aromatic hydrocarbons, liquid fuels (naphtha, kerosine and diesel), methanol and ammonia.
Manufacture of chemicals from biomass by fermentation
Chemicals such as ethanol and butanol are currently produced by fermentation processes that are discussed in detail in the unit on Biofuels.
Manufacture of chemicals from biomass by thermochemical processes
There are two main routes using thermochemical processing:
- One involves heating biomass to a high temperature and under pressure in a controlled amount of oxygen which leads to the production of synthesis gas (a mixture of carbon monoxide and hydrogen) (Figure 2, route 1). This process is known as gasification. Synthesis gas can then be converted into many important industrial compounds. Gasification leading to kerosine and diesel fuels is discussed in the unit on Biofuels.
- The second method again involves heating the biomass to a high temperature, but in this case in the absence of air. This process is known as pyrolysis. In order to form useful products, the reaction time must be very short, otherwise the major product will be carbon (char). This process is thus called fast pyrolysis and the major product is an oil known as bio-oil (Figure 2, route 2). The production of bio-oil is discussed in more detail in the unit on Biofuels.
Biorefineries-possible synthetic routes
Figure 2 shows possible synthetic routes and products that could be made from biomass in a biorefinery. The routes and products that follow each of the main initial processes, fermentation, gasification and pyrolysis are described below.
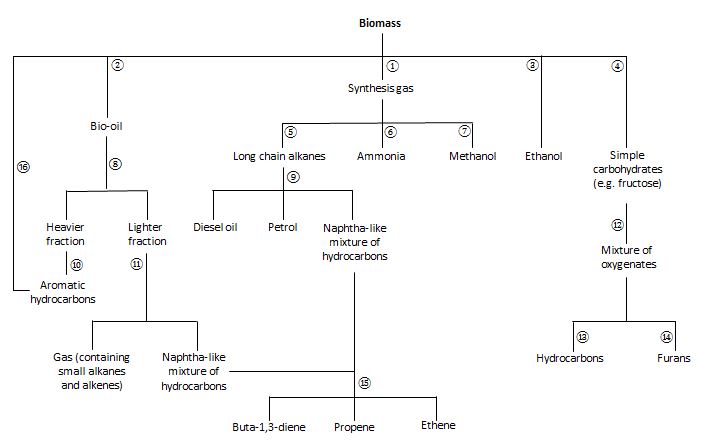
Figure 2. A process flowchart of a biorefinery in the future
| Key | |||
|---|---|---|---|
| 1 | High temperature gasification | 9 | Hydrocracking |
| 2 | Fast pyrolysis | 10 | Heated catalyst |
| 3 | Fermentation | 11 | Catalytic cracking |
| 4 | Acid hydrolysis | 12 | Bioforming |
| 5 | Fisher-Tropsch (Shell Middle Distillate) process | 13 | Catalytic reduction |
| 6 | Haber process | 14 | Dehydration |
| 7 | High temperature and pressure with a catalyst | 15 | Steam cracking |
| 8 | Distillation | 16 | Heating sawdust over a catalyst |
Manufacture and uses of bioethanol (Figure 2, route 3)
The fermentation of sugars from various forms of biomass is described in the unit on Biofuels. While the overwhelming proportion of ethanol production is used as a fuel, an increasing amount is being used as a chemical intermediate, for example in the production of ETBE, ethyl t-butyl ether, like ethanol itself an important additive to petrol to improve its octane rating. Bioethanol is also being used to make bio-based poly(ethene) (bio-based polyethylene).
Manufacture of synthesis gas (carbon monoxide and hydrogen) from biomass (Figure 2, route 1)
Any solid biomass including for example agricultural, city and industrial waste can be used to make synthesis gas using techniques similar to its production from coal. More recent developments includes a plant in the Netherlands, which is using liquid propane-1,2,3-triol (glycerol), a by-product from the production of biodiesel, from animal fats and vegetable oils.
Manufacture of and uses of methanol from biomass
There are several ways in which methanol is made from biomass.
Synthesis gas formed from gasification of biomass can be converted into methanol (Figure 2, route 7).
Although much of the methanol is added to petrol, it can also be converted to propene by the MTP process (Figure 3, route 9) and thus to poly(propene) (polypropylene). The methanol can also be used as the feedstock for the production of a range of other chemicals, particularly in the plastics industry.
Manufacture of ammonia from biomass
Hydrogen within synthesis gas formed by gasification of biomass, can be converted into ammonia (Figure 2, route 6), by addition of nitrogen from air, and is used mainly to make fertilizers.
Manufacture of bio-based ethene (bio-based ethylene) and bio-based poly(ethene) (bio-based polyethylene)
(a) Manufacture of bio-based ethene via fermentation
A new use for bioethanol is its dehydration to ethene, an important chemical with many applications (Figure 3, route 6). Plants in the south-east of Brazil are been built for this process:
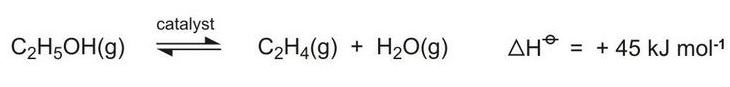
As can be seen from the equation above, the equilibrium is favoured by high temperatures and hindered by higher pressures and water vapour. Ethanol vapour is passed over fixed beds of the catalyst at temperatures in the range of 600-750 K and conversion rates of over 99.9% have been achieved. Higher temperatures, however, lead to the formation of ethanal (acetaldehyde). The catalyst used is based on a mixture of magnesium, aluminium and silicon oxides.
(b) Polymerization of bio-based ethene
Ethene from this plant is polymerized (Figure 3, eroute 12) to produce the polymer by one of the methods described in the unit on poly(ethene).
This contrasts with the current route to ethene, the steam cracking of various fractions from the distillation of oil, a finite resource.
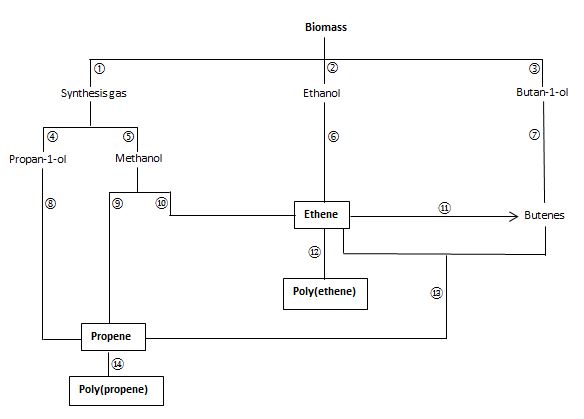
Figure 3 Manufacture of poly(ethene) and poly(propene) from biomass
| Key | |||
|---|---|---|---|
| 1 | High temperature gasification (also Figure 2, route1) | 8 | Dehydration |
| 2 | Fermentation (also Figure 2, route 3) | 9 | MTP process |
| 3 | Fermentation | 10 | MTO process |
| 4 | Heat with ethanol over a catalyst | 11 | Dimerization |
| 5 | High temperature and pressure with a catalyst (also Figure 2, route 7) | 12 | Polymerization |
| 6 | Dehydration | 13 | Heat over a catalyst |
| 7 | Dehydraton | 14 | Polymerization |
Manufacture of bio-based propene (bio-based propylene) and poly(propene) (polypropylene)
(a) Manufacture of bio-based propene via-fermentation
There are several methods being developed to produce propene from biomass. For example:
(i) via ethene and butene
One system that is being used is the metathesis reaction between ethene and butene to form propene, for example:
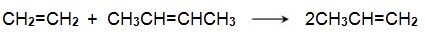
Bio-based ethene can be obtained by dehydration of bioethanol using a silica/alumina or alumina catalyst (Figure 3, route 6). The butenes (but-1-ene and but-2-ene) can be produced by either dehydration of biobutanol (Figure 3, route 7) or by dimerization of bioethene (Figure 3, route 11).
The dimerization of ethene to but-1-ene is carried out by passing heated ethene over a zeolite impregnated with a transition metal complex. A variety of complexes of rhodium, titanium and other metals are used:

A mixture of ethene and butene is then heated and passed over a solid catalyst based on organic compounds of molybdenum(IV) and tungsten(IV) (the Schrock catalysts) and organo-ruthenium (II) compounds (the Grubbs’ catalysts), in a fixed-bed reactor (Figure 3, route 13):
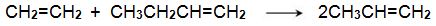
Small amounts of coke are deposited on the catalyst and are removed from time to time by passing heated air through the reactor.
The catalysts are also used in the Shell Higher Olefines Process (SHOP). Richard Schrock and Richard Grubbs were among three joint winners of the 2005 Nobel Prize for Chemistry.
(ii) The MTP Process
Another way to produce propene is via methanol (produced from biomass via synthesis gas), which is an example of the MTO (Methanol To Olefins) process. (Olefin is the older name for the homologous series, alkenes). Methanol can be converted into high purity ethene and propene via dimethyl ether (Figure 3, routes 10 and 9). Methanol vapour is passed over alumina at ca 600 K. An equilibrium mixture of methanol, dimethyl ether and steam is produced, containing about 25% methanol:
.jpg)
This mixture of gases is then passed over a bed of a zeolite in a form that encourages high selectivity towards alkenes with numbers of carbon atoms from 2 to 8. However, by using a zeolite treated with acid, almost all the alkene produced is propene, this being known as the MTP (Methanol To Propene) process (Figure 3, route 9). The propene is purified by cooling it to a liquid and then subjecting the liquid to fractional distillation.
As discussed in the section on methanol, a similar process is used to make hydrocarbons used in gasoline, the MTG (Methanol to Gasoline) process.
(iii) via propan-1-ol
Another method of producing bio-based propene is via syngas and propan-1-ol. Synthesis gas (carbon monoxide and hydrogen) is used to convert bioethanol to propan-1-ol (Figure 3, route 4):

The reaction is catalysed by a ruthenium-cobalt complex salt. A molybdenum-based catalyst is also being used as it is more resistant to poisoning by sulfur-containing impurities in the feedstock.
Subsequently, propan-1-ol is dehydrated to propene (Figure 3, route 8):

(b) Manufacture of bio-based poly(propene) (bio-based polypropylene)
The propene from this plant is then polymerized to produce the polymer by one of the methods described in the unit on poly(propene) (Figure 3, route 14).
Manufacture of 1,4-dimethylbenzene (p-xylene)
Much research is being devoted to producing hydrocarbons such as 1,4-dimethylbenzene (p-xylene), from biomass. Here the overall strategy is to remove the oxygen atoms in complex molecules in biomass, by first converting biomass to bioethanol and dehydrating it to bio-based ethene (ethylene).
The alkene can be converted into a trimer, hex-1-ene, when passed over a chromium-based catalyst:
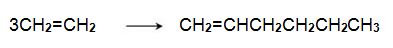
Hex-1-ene reacts with another molecule of ethene on an iridium-based catalyst to form 3,6-dimethylcyclohexene,
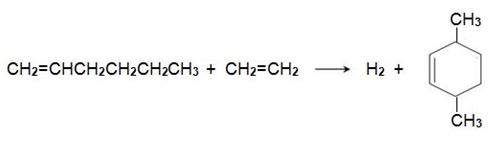
which is dehydrogenated to 1,4-dimethylbenzene, when passed over aluminium oxide impregnated with platinum:
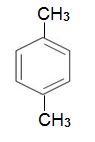
The aromatic hydrocarbon is the starting point for the manufacture of polyesters.
Manufacture of fuels
Synthesis gas is converted into a hydrocarbon wax (a mixture of long-chain alkanes) by heating it and passing the vapour over a cobalt catalyst (the Fischer-Tropsch process) (Figure 2, route 5). The Shell Middle Distillate synthesis (SMDS) is a modern development of this process. The hydrocarbon waxes are subsequently catalytically cracked with excess hydrogen (hydrocracking) (Figure 2, route 9) to form smaller alkanes, for example:

These alkanes can be used in liquid fuels, diesel, kerosine and naphtha, the choice depending on their volatility.
Smaller amounts of light gases are also produced in the Fischer-Tropsch reactor and the hydrocracker (for example, ethane and propane). These can either be recycled back to the gasifier or used for heat and power production in the biorefinery.
There is increasing interest in producing aromatic hydrocarbons from biomass, for use as a chemical feedstock and as a fuel (aromatic hydrocarbons have a high octane rating).
Although the principal use of bio-oil, produced by the fast pyrolysis of biomass, is as a fuel, it is also a promising source of chemicals. The bio-oil can be separated by distillation into two components, a lighter fraction and a heavier one (Figure 2, route 8). The lighter fraction can be catalytically cracked (Figure 2, route 11), in a similar way to the cracking of gas oil, to yield a gas, containing alkanes and alkenes and a naphtha-like liquid which can be steam cracked to yield ethene, propene and buta-1,3-diene (Figure 2, route 15). These are all major feedstocks for a variety of important chemicals.
The heavier fraction contains substituted phenols and aromatic oligomers (small polymers of 3-8 monomers) and on passing the liquid and their vapours over a heated zeolite catalyst in a fixed-bed reactor a high concentration of aromatic hydrocarbons, benzene, methylbenzene (toluene) and the three methylbenzenes (xylenes), known as BTX, are recovered (Figure 2, route 10). Alternatively the vapours can be mixed with hydrogen and passed over a catalyst such as a cobalt- molybdenum sulphide on alumina.
In another process that is being developed, finely divided biomass (for example, sawdust) is passed as a fluid, over a heated zeolite catalyst, in the absence of air (Figure 2, route 16). This produces a mixture of benzene, methylbenzene (toluene) and dimethylbenzenes (xylenes) as well as other hydrocarbons.
In another new development, biomass is heated with acid and the complex carbohydrates (for example, starch) are hydrolysed to simpler carbohydrates (for example fructose and glucose) (Figure 2, route 4). These are purified and their aqueous solutions undergo a process, known as chemocatalysis or bioforming (Figure 2, route 12). They are converted, catalytically, in the aqueous phase, to form a mixture of aliphatic and cyclic oxygenates as well as hydrogen. The mixture can then be reduced with hydrogen to hydrocarbons , and passed over a zeolite catalyst to form a mixture that is similar to a gasoline feedstock with a high aromatic content, and thus a high octane rating (Figure 2, route 13).
Manufacture of other chemicals
Examples of other chemicals produced by fermentation are described in other units. These include the biofuels, such as biobutanol and biodiesel, and propane-1,3-diol and 2-hydroxypropionic acid (lactic acid), both used to make polymers.
A very wide range of chemicals can be produced in chemocatalytic (bioforming) reactions (Figure 2, route 14).
One chemical that is exciting much interest is hydroxymethylfurfural, HMF,
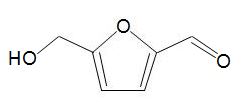
formed by dehydration of simple carbohydrates such as fructose. A catalyst with acid groups and which is water-tolerant such as a zeolite is used. HMF can be converted into dimethylfuran,
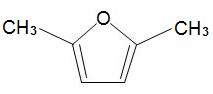
which is widely used as a solvent and could also be used as a fuel in place of ethanol.
HMF can be oxidized to a dicarboxylic acid,
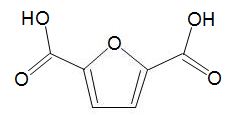
which can be used in place of benzene-1,4-dicarboxylic acid (terephthalic acid) and co-polymerised with a diol to make a polyester with similar properties to polyethylene terephthalate (PET).
An analogy with oil refineries
The future biorefinery will look very similar to the present day oil refinery. The components in crude oil vary from dissolved gases to very heavy bituminous tars and the first step is to separate these by distillation. These are then subjected to a variety of chemical and physical processes to change their composition and to purify them. These include the cracking of heavy oils to lighter oils, the isomerisation of straight chain alkanes to the more useful branched alkanes, the reforming of straight chain alkanes to aromatic hydrocarbons and the removal of polluting sulfur compounds.
Biorefineries, on the other hand, will be based on the oxidation or pyrolysis of biomass and chemocatalytic reactions to produce the simpler molecules in syngas or long-chain hydrocarbons from bio-oil. These will, like the components from the distillation of crude oil, be processed by chemical and physical means to the same end-products. The biorefinery will also be producing chemicals by fermentation. Oil refineries and, in the future, biorefineries, operate on a very large scale, are highly integrated and extract value from all fractions of crude oil and biomass thus allowing the products to be made efficiently at low cost.
Date last amended: 7th September 2016
